Medical Care
The diagnosis of retinitis pigmentosa (RP) can be overwhelming to many patients. Although therapies are limited, physicians should emphasize the therapies that are available to help patients. Perhaps, most importantly, it is essential to help patients maximize the vision they do have with refraction and low-vision evaluation. Many devices are available to help patients with night vision difficulties, and most low-vision clinics are aware of these devices.
The authors believe that patients should have annual examinations, including visual field testing and periodic (every 5 y) ERG evaluations. Changes in examination findings can help guide patients in their activities and can help with prognosis. Often, these examinations can provide reassurance that the changes are slow. In addition, regular examinations can ensure patients have appropriate community and legal assistance. Finally, as new therapies emerge, routine evaluation can keep patients informed of clinical trials and new treatments.
Gene therapy has been approved by the FDA for patients with confirmed biallelic (ie, having both a paternal and a maternal mutation) RPE65 mutation-associated retinal dystrophy.
Vitamin A/ beta-carotene
Antioxidants may be useful in treating patients with RP, but no clear, prospective evidence in favor of vitamin supplementation yet exists.
A recent comprehensive epidemiologic study concluded that very high daily doses of vitamin A palmitate (15,000 U/d) slow the progress of RP by about 2% per year. The effects are modest; therefore, this treatment must be weighed against the uncertain risk of long-term adverse effects from large chronic doses of vitamin A.
Annually check liver enzymes and vitamin A levels. Beta-carotene doses of 25,000 IU have been recommended.
Docosahexaenoic acid (DHA)
DHA is an omega-3 polyunsaturated fatty acid and antioxidant.
Studies have shown a correlation of ERG amplitudes with patients' erythrocyte-DHA concentration. Others studies reported trends of less ERG change in patients with higher levels of DHA. Nutritional intake of omega-3 fatty acids may affect the rate of decline of visual acuity (see Diet), although further clinical trials must be done to determine DHA benefit.
Lutein/zeaxanthin
Lutein and zeaxanthin are macular pigments that the body cannot make but instead come from dietary sources. Lutein is thought to protect the macula from oxidative damage, and oral supplementation has been shown to increase the macular pigment.
A National Institutes of Health (NIH) clinical trial, the Age-Related Eye Disease Study II (AREDS II), is beginning to test the effectiveness of lutein and zeaxanthin to slow age-related macular degeneration. Their ability to prevent cone photoreceptor cell death (such as what occurs in RP) has not been shown.
Doses from 6-20 mg per day have been recommended.
Valproic acid
Oral valproic acid (VPA) initially was found show benefit in small clinical studies; however, a recent randomized prospective trial enrolled 90 ADRP patients and gave them oral VPA 500 mg to 1000 mg daily for 12 months or placebo. The patients who recieved VPA had worse outcomes in terms of visual fields than the placebo group. [15] Therefore at this time we do not recommend VPA use for patients outside of clinical trials.
Calcium channel blockers
Calcium channel blockers, such as diltiazem, are medications commonly used in cardiac disease. Calcium channel blockers have shown some benefit in some animal models of RP, but they have been ineffective in other models. No current recommendations exist regarding the use of calcium channel blockers in patients with RP.
Medications with potential adverse effects in RP
Isotretinoin (Accutane): A medication used to treat acne has been reported to worsen night vision, ERG response, and dark adaptation. As its safety in patients with RP is not known, many physicians do not recommend isotretinoin use for their patients.
Sildenafil (Viagra): A medication to treat erectile dysfunction has been shown to cause reversible ERG and vision changes. Sildenafil is an inhibitor of PDE5 and less so PDE6. Mutations of the PDE6 gene are known to cause autosomal recessive RP. Therefore, physicians have suggested that this medication may not be safe for patients with RP, including carriers of the PDE6B gene mutation. Some users of sildenafil have experienced blue photopsias, suggesting that the drug is active in the retina at a physiological level.
Vitamin E: Reports have suggested that high doses of vitamin E (400 U/d) may be modestly deleterious in patients with RP. While doses as high as 800 IU/d have been recommended by some authors, the authors of this article recommend avoiding additional supplementation with vitamin E until further studies are conducted.
Other medications
Although doses of 1000 mg/d ascorbic acid have been recommended, no evidence exists that ascorbic acid is helpful.
Although bilberry is recommended by some practitioners of alternative medicine in doses of 80 mg, no controlled studies exist that document its safety or efficacy in treating patients with RP.
In patients who present with antiretinal antibodies, immunosuppressive agents (including steroids) have been used with anecdotal success.
Surgical Care
Cataract extraction
Cataract surgery can often be beneficial in the later stages of RP. Bastek et al studied 30 patients with RP; 83% of them improved by 2 lines on the Snellen visual acuity chart with cataract surgery. [16] Perioperative use of corticosteroids is recommended to prevent postoperative cystoid macular edema. Educating patients about reasonable expectations of cataract surgery is essential.
Growth factors
Ciliary neurotrophic factor (CNTF) has been shown to slow retinal degeneration in a number of animal models.
Two Phase II clinical trials have been conducted using an encapsulated form of RPE cells producing CNTF (Neurotech) for patients with Usher syndrome and RP. These encapsulated cells must be surgically placed into the eye. The results from this study, showed some evidence of retinal thickening after 1 year of treatment; however visual improvement or visual field improvment was not seen in either trial. [17] Long-term followup has continued to see no improvement.
Transplantation:
Cell-based therapies /transplantation have been studied for many years for the treatment of retinal disease. Transplanted cells many provide supportive benefit to the host cells to prevent further degnereation and/or stimulate some regeneration of the retina. Alternatively, the transplanted cells themselves may be able to integrate directly into the retina to function and replace the areas of pathologic damage. [18] The potential source of therapeutic cells include many potential types including: adult bone marrow–derived stem cells, umbilical cord-derived stem cells, pluripotent stem cell-derived photoreceptor or retinal pigment epithelium (RPE) cells. All these types of cells have been and continue to be studied in preclinical and clinical trials for RP.
Many clinical trials have already have had encouraging results. An early encouraging human clinical trial tested stem-cell–derived therapy for people with age-related macular degeneration and Stargardt disease. In this study, the cells derived from stem cells are differentiated into cells with an RPE phenotype and then injected under the retina during vitrectomy surgery. After two year followup they found that the RPE cells did survive in the subretinal space, but they did not see signficant benefit to the vision of patients. [19, 20] Two other companies currently have clinical trials underway using retinal progenitor cells (RPC) for patients with RP. The California company JCytes has sponsored a clinical using an intravitreal injection for RPCs to treat RP patients, where as the Reneuron has taken a different approach by delivering the RPC cells under the retina for RP patients. (clinicaltrials.gov: Trials NCT03073733 and NCT02464436)
Transplantation of stell-derived cells and adult retinal tissue has also been studied in clinical trials without significant benefit or reproducibly improving patients’ vision. Small patches of retinal or RPE tissue have been transplanted, and this technique could be helpful in the following RP forms: when RP is based on an RPE defect, when RP with primary defects exists in the outer segments, if the disease is driven by an overload of the phagocytic activity of the RPE, or if the RPE cannot provide sufficient nutritional support to the outer segments.
Retinal prosthesis:
Artificial vision for patients without any vision has only recently become a reality after years of research and investment. One effective approach uses a retinal prosthesis or phototransducing chip placed on the retinal surface. A digital camera placed in glasses can then transmit a stimulus to the intraocular chip, which electrically stimulates the retina in a pattern mimicking the image transmitted from the glasses, thereby giving the patient an electrically produced image to the ganglion cell layer of the retina. Preclinical trials in animal models demonstrated long-term stability. [21]
The US Food and Drug Administration (FDA) has approved the Argus II Retinal Prosthesis System, for adults aged 25 years or older with advanced RP. [2] Although this device will not restore vision to patients, it replaces the function of degenerated cells in the retina and may improve a patient’s performance of basic activities by improving their ability to perceive images and movement. [2] The implant includes a small video camera, transmitter mounted on a pair of eyeglasses, video processing unit (VPU), and an implanted retinal prosthesis (artificial retina). The VPU transforms images from the video camera into electronic data that are wirelessly transmitted to the retinal prosthesis. About two thirds of patients had no adverse events related to the device or the procedure; however, over one third of patients had a total of 23 serious adverse events, including conjunctival erosion, dehiscence, retinal detachment, inflammation, and hypotony. [2]
Many researchers and companies continue to attempt to give patients vision through technological advances. For example, Chow et al placed subretinal microphotodiodes (prosthesis) in patients with severe RP. [22] These patients had subjective improvement; however, the improvement was delayed and occurred in retinal areas outside of where the chip was placed. Therefore, the effect was thought to be an indirect benefit to adjacent cells. Other studies are trying to bypass the eye and directly stimulate the occipital lobe of the brain to provide artificial vision to patients. Many other types of retinal prosthesis continue to be studied including: Retina Implant Alpha-IMS, Retina Implant Alpha-AMS, Photovoltaic Retinal Implant (PRIMA) bionic vision system, EPIRET3 and many others.
Gene therapy:
As noted previously, there are over 70 genetic mutations that can cause various types of RP. Only recently, gene therapy has proven to successfully treat some forms of RP and currently has many active clinical trials, with the hope to replace the defective protein by using DNA vector (eg, adenovirus, lentivirus). [23]
Mutations in the human retinal pigment epithelial 65-kd protein (RPE65) gene are one of the causes of retinitis pigmentosa and Leber congenital amaurosis (a form of retinitis pigmentosa at birth). Voretigene neparvovec (Luxturna) is the first gene therapy approved by the FDA that targets the RPE65 gene. It is indicated for confirmed biallelic (ie, having both a paternal and a maternal mutation) RPE65 mutation-associated retinal dystrophy in patients aged 1 year and older. It is an adeno-associated virus vector-based gene therapy designed to deliver a normal copy of the gene encoding RPE65 to cells of the retina.
Approval was based on improvement as measured by multiluminance mobility testing among treated patients (n = 20) compared with those who received placebo (n = 9) (p = 0.0013). [24] Overall, improvements in ambulatory navigation, light sensitivity, and visual field were maintained up to 3-4 years. [25]
Gene therapy was successful in providing the missing protein to a dog with Leger congenital amaurosis (LCA). Using adeno-associated virus (AAV), the Briard dog with RPE65 mutations after treatment had 20% of its RPE cells express the functional protein, thereby allowing the dog to see. This was also effective in a mouse model of Leber congenital amaurosis.
These prospective clinical trials were proven to successfully treat patients with the RPE65 mutation. [26] This has led to an FDA-approved treatment for these patients. Because of the wide heterogeneity of defects in RP, gene therapy must be targeted specifically to each mutation. Subsequently, many trials have also begun for other genes that cause RP – including ABCA4, CHM, RS1, MYO7A, CNGA3, CNGB3, ND3, MERTK, and RPGR. (clinicaltrials.gov)
Consultations
Clearly, RP is associated with several systemic diseases. Because of the severity of the systemic illness and its early presentation in most patients, the ophthalmologist may act as the consultant to an internist.
All patients should see their primary care specialist to ensure they are otherwise in good health and that their vision changes are not related to any other systemic conditions.
Low-vision specialists:
Low-vision specialists are eye care professionals who aim to improve the lives of patients with low vision through careful evaluation and the provision of testing, training, devices, assistance, and wisdom. The advances in technology and visual assistance for patients with low vision has continued to improve every year. We highly recommend that all patients with RP who are struggling with any decrease in vision should see the low vision specialist / clinic for assistance.
Audiology consults should be considered for patients with hearing loss or for those patients with known Usher syndrome.
Genetic Counseling:
Genetic testing and counseling is becoming increasingly valuable as the understanding of RP increases. Identification of the patient’s genotype offers several advantages. First, it confirms the genetic cause of the condition. In addition, it can occasionally help determine prognosis and may likely prove to be important for future therapy choices. Genetic counseling is very helpful to guide patients on the hereditary nature of their disease and the mode of inheritance. This counseling can help the patients with their future plans, such as pregnancy, job choices, and medical treatments. The availability and quality of genetic testing has improved greatly over that past several years, and some organizations like the Foundation Fighting Blindness are currently offering it for free for those patients enrolled in their Retina Tracker Program.
Moreoever, psychological counseling should be made available to those patients when appropriate.
Diet
Many practitioners recommend a well-balanced diet with adequate leafy green vegetables that contain the aforementioned supplements in nontoxic doses.
For patients receiving vitamin A palmitate, a diet rich in omega-3 fatty acids may slow the rate of decline of visual acuity. [27]
Activity
Light exposure
Stressful light exposure, which generates free radicals and strains the regenerative capacity of the eye, might put dystrophic retinas at a disadvantage. However, little direct or epidemiologic evidence exists that the disease is modified by light.
A specific form of RP, the Pro23His mutation in rhodopsin, has been shown to have increased retinal damage with increased light exposure.
UV-absorbing lenses are recommended, particularly in rhodopsin mutation varieties of RP, and patients with cone degeneration frequently benefit from tinted lenses.
Complications
Treatment for the Complication of Cystoid Macular Edema:
Many medications and anti-inflammatories have shown some benefit for the treatment of this complication:
Carbonic Anyhydrase Inhibitors (CAIs):
Cystoid macular edema (CME) is not an uncommon complication of RP that can reduce vision, which as many as 40% of RP patients experience. Of the many therapies tried, oral acetazolamide has shown the most encouraging results with some improvement in visual function. Studies by Fishman et al and Cox et al have demonstrated improvement in Snelling visual acuity with oral carbonic anhydrase inhibitor acetazolamide for patients who have RP with macular edema. [28] Recent clinical studies continue show both topical CAIs such as dorzolamide and oral acetazolmide have helped pateint who have the complication of CME with RP. [10, 11] Adverse effects, including fatigue, renal stones, loss of appetite, hand tingling, and anemia, may limit its use.
Corticosteroids:
Intravitreal triamcinolone and dexamethsone implant have both shown to be effective in small case series for RP patients with CME. [12, 13] Many have also see benefit from topical corticosteroids.
Anti-Vascular Endothelial Growth Factor (anti-VEGF):
Recent studies have found that CME has also been sucessful treated in RP patients with intravitreal anti-VEGF such as bevacizumab. [14]
Prevention
There is no proven therapy to help prevent the progressive degeneration found in RP; however gene therapy has shown the greatest promise, and anti-oxidative supplements seem to slow the rate. Hopefully future studies will show cell therapy or neww medications can slow or reverse the progression.
-
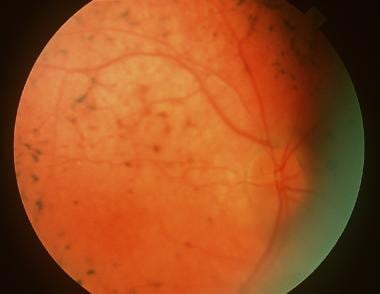
Usher syndrome with typical retinitis pigmentosa appearance.
-
Choroideremia.
-
Bull's eye maculopathy seen in cone dystrophy.
-
Polydactyly seen in Bardet-Biedl syndrome (associated with retinitis pigmentosa).
-
Cone dystrophy.
-
Gross pathology of an eye in a man with retinitis pigmentosa.
-
Leber congenital amaurosis.
-
Female carrier of choroideremia.
-
Representative electroretinograms of patients with healthy eyes, rod-cone dystrophy, and congenital stationary night blindness. Courtesy of Dr. Nusinowitz, Jules Stein Eye Institute.
-
Representative electroretinograms of patients with healthy eyes and X-linked retinoschisis. Courtesy of Dr. Nusinowitz, Jules Stein Eye Institute.
-
Retinitis pigmentosa pigmentation pattern demonstrated with ultrawide fundus imaging using the scanning laser ophthalmoscope (Optomap; Optos PLC, Dunfermline, Scotland, United Kingdom).
-
Fellow eye of same patient as in the image above, again demonstrating a typical retinitis pigmentosa pigmentation pattern demonstrated with ultrawide fundus imaging using the scanning laser ophthalmoscope (Optomap; Optos PLC, Dunfermline, Scotland, United Kingdom).
-
Higher resolution image of typical bone spicule formation.
-
Cone dystrophy demonstrating typical central macular atrophy found in this condition.
-
Retinitis pigmentosa, rubella, a history of retinal detachment, and syphilis all may result in a hyperpigmented retinal pigment epithelium (RPE) with bone spicule appearance, restricted visual field and/or poor vision, and atrophic vessels.
-
Retinitis pigmentosa progresses over decades. Associated cataract also is relevant, as seen in this image.
-
Pigmentary changes are not always seen in retinitis pigmentosa but frequently are observed, as in this patient with Alström disease.
-
Genetic screening may be helpful in identifying patients who are at risk, in counseling, and in directing treatment as new knowledge is acquired. Some varieties of retinitis pigmentosa may have increased vulnerability to environmental hazards; for example, one might avoid light exposure in some rhodopsin mutations or sildenafil in phosphodiesterase mutations. Patients with retinitis pigmentosa may have other findings. This patient with Alström disease shows acanthosis.