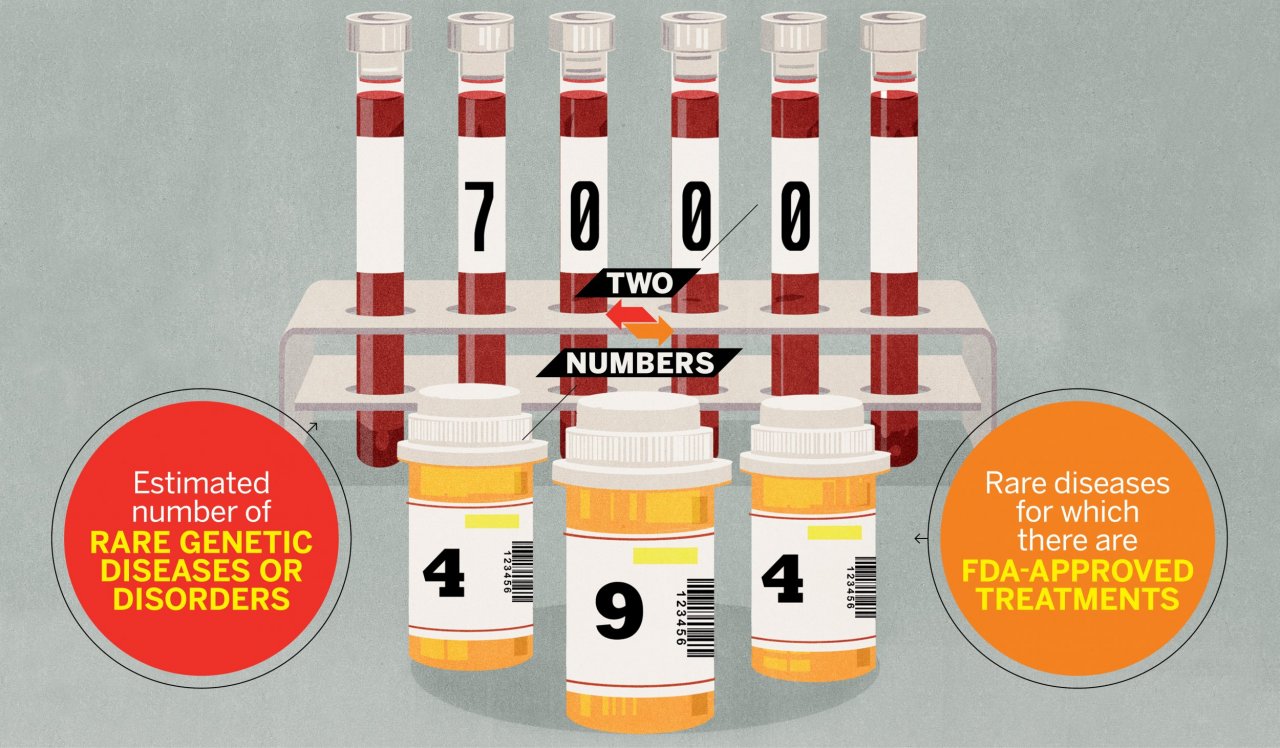
Approximately 30 million people in the U.S. and 350 million people worldwide are living with conditions so rare they are understudied, untreated and, in some cases, even unrecognized. In the U.S., a disease is considered rare if it afflicts fewer than 200,000 patients, and there are an estimated 7,000 rare diseases, many of which are hereditary and caused by genetic mutations. They are often degenerative to one or more systems of the body, and many are fatal.
Pharmaceutical companies have historically been slow to develop treatments for these complex conditions. The money is in blockbuster drugs for top killers like heart disease, not in treatments that may benefit only a few hundred patients. This reality leaves millions of people with these conditions—called orphan diseases—without options. Half of these patients are children who won't live long enough to celebrate their fifth birthday.
In 1983, Congress addressed this challenge when it passed the Orphan Drug Act, meant to encourage drug research and development for rare diseases. Under the law, pharmaceutical companies that develop an orphan drug can market and sell it exclusively for seven years. Additionally, the law required the U.S. Food and Drug Administration (FDA) to help design clinical trials that allow patients easier access to experimental treatments; the idea was to speed up the approval process.
"At that point in time, it was felt that investigators didn't really understand how to develop a study with a small population, in which patients were spread out within a relatively large area," explains Stephen Groft, senior adviser to the director of the National Center for Advancing Translational Sciences, a division of the National Institutes of Health (NIH).
According to the FDA, 10 treatments for rare diseases were approved in the decade before the law passed. In the first 10 years after the law passed, the FDA approved 93 therapeutic drugs for orphan designation. Currently, there are 494 total FDA-approved treatments for orphan diseases. Groft says the NIH is currently supporting 9,400 projects with $3.6 billion dedicated to advancing progress in this area of medical research.
In recent years, tenacious families and patients who lead fundraising efforts and communicate directly with the NIH, FDA and scientists have helped push along much of the research on rare diseases. "The patients, families and patient advocacy groups have really become research partners, along with the scientists and pharmaceutical companies," says Groft.
The broader medical science move toward targeted treatment should help too. The completion of the Human Genome Project in 2003 has also benefited this patient population, since many rare diseases are the result of faulty DNA. In addition, government-led efforts such as President Obama's Precision Medicine Initiative (unveiled earlier this year) and the 21st Century Cures Act (legislation approved in May by a committee of the U.S. House of Representatives) are meant to encourage collaboration, patient involvement and push the idea that modern medicine isn't a "one-size-fits-all" venture.
But in the meantime, though many families continue to hold out hope that a cure will be found overnight, Groft says in many cases researchers simply need more time. "I think we often find the science isn't ready yet," says Groft. "We don't understand the disease enough."