Two teams of scientists have developed new ways of stimulating neurons with nanoparticles, allowing them to activate brain cells remotely using light or magnetic fields. The new methods are quicker and far less invasive than other hi-tech methods available, so could be more suitable for potential new treatments for human diseases.
Researchers have various methods for manipulating brain cell activity, arguably the most powerful being optogenetics, which enables them to switch specific brain cells on or off with unprecedented precision, and simultaneously record their behaviour, using pulses of light.
This is very useful for probing neural circuits and behaviour, but involves first creating genetically engineered mice with light-sensitive neurons, and then inserting the optical fibres that deliver light into the brain, so there are major technical and ethical barriers to its use in humans.
Nanomedicine could get around this. Francisco Bezanilla of the University of Chicago and his colleagues knew that gold nanoparticles can absorb light and convert it into heat, and several years ago they discovered that infrared light can make neurons fire nervous impulses by heating up their cell membranes.
They therefore attached gold nanorods to three different molecules that recognise and bind to proteins in the cell membranes – the scorpion toxin Ts1, which binds to a sodium channel involved in producing nervous impulses, and antibodies that bind the P2X3 and the TRPV1 channels, both found in dorsal root ganglion (DRG) neurons, which transmit touch and pain information up the spinal cord and into the brain.
The researchers added these particles to DRG neurons growing in Petri dishes, so that they would bind to the cells displaying the relevant proteins on their surface. They then exposed the cells to millisecond pulses of visible light, which heated up the particles, causing the cells to fire nervous impulses in response. This was possible not only in isolated neurons but also in slices of tissue from the rat hippocampus. In both situations, the particles stayed firmly in place when added in low concentrations, allowing for repeated stimulation of the cells for over half an hour.
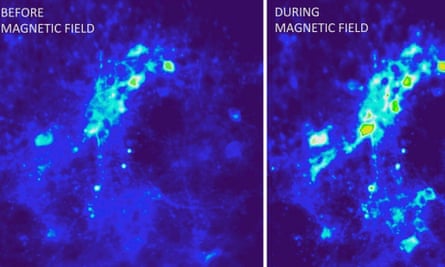
Polina Anikeeva’s team at the Massachusetts Institute of Technology adopted a slightly different approach, using spherical iron oxide particles that give off heat when exposed to an alternating magnetic field.
First, they injected a virus carrying the TRPV1 gene into the ventral tegmentum of mice, so that neurons would take up the virus and express the gene, making them sensitive to heat. A month later, they injected the nanoparticles into in the same part of the brain, and then applied magnetic fields to it. This made the nanoparticles give off heat enough to activate the TRPV1 channels, causing the neurons to fire long trains of nervous impulses.
Neurons engulf iron oxide nanoparticles, and the researchers found that the particles they injected persisted in the animals’ brains, so that they could continue to activate cells in the ventral tegmentum for up to a month later, while causing less tissue damage than implantable stainless steel electrodes.
Both methods are quite limited in their specificity. The gold nanoparticles bind only to the multiple cell types that express the sodium channel, P2X3, or TRPV1, while the TRPV1 virus and iron oxide particles enter cells at random around the injection site. This is easily solved, as nanoparticles can be conjugated to just about any molecule, but while both methods can activate neurons, neither can inhibit them, and it’s not at all clear how they might be tweaked in order to do so.
Nanoparticles are already being used in other fields. They can, for example, target and destroy malignant cells, and therefore show promise in cancer therapy. More recently, some researchers have exploited their ability to sneak through the blood-brain barrier, and have used them to visualise and reduce stroke damage and inflammation in rats.
Although still in the experimental stages, research like this may eventually allow for wireless and minimally invasive deep brain stimulation of the human brain. Bezanilla’s group aim to apply their method to develop treatments for macular degeneration and other conditions that kill off light-sensitive cells in the retina. This would involve injecting nanoparticles into the eye so that they bind to other retinal cells, allowing natural light to excite them into firing impulses to the optic nerve.
References: Carvalho-de-Souza, J. L., et al. (2015). Photosensitivity of Neurons Enabled by Cell-Targeted Gold Nanoparticles. Neuron, DOI: 10.1016/j.neuron.2015.02.033
Chen, R., et al. (2015). Wireless magnetothermal deep brain stimulation. Science, DOI: 10.1126/science.1261821 [PDF]
Comments (…)
Sign in or create your Guardian account to join the discussion