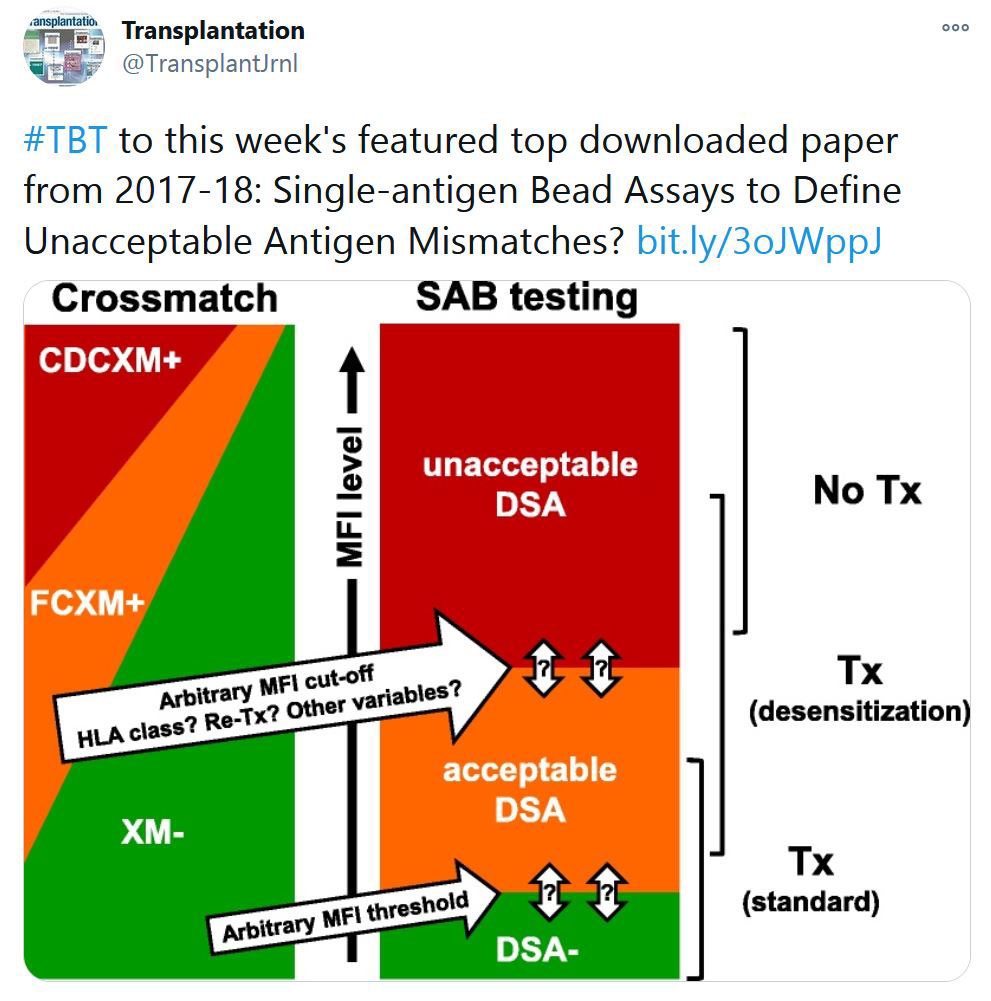
Members were emailed their invoices a month ago.
If you are not a member click the Join TTS button below!
CORONAVIRUS (COVID-19) UPDATES
«HOT OFF THE PRESS»
RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
Selected Publications by TTS Education Committee. This week's selection made by Enver Akalin.
TTS and TTS Sections News
Latest Video Content Posted

Videos from the Congress are now available to TTS Members.

SPLIT 2020 Meeting videos are now available to TTS & SPLIT Members.

Beta Cells Summit videos are now available to TTS & IPITA Members.
Dec 3 - COVID-19: ORGAN DONATION & TRANSPLANT TOWN HALL # 4
Nov 22 - FUNDAMENTALS TO COMPLEX DECISIONS WITH KIDNEY TRANSPLANTATION - Milagros Samaniego Picota
IN THE NEWS
UPCOMING MEETINGS AND ANNOUNCEMENTS
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada