protein
(redirected from Glial fibrillary acidic protein)Also found in: Dictionary, Thesaurus, Medical, Acronyms, Wikipedia.
protein
Types of Proteins
A protein molecule that consists of but a single polypeptide chain is said to be monomeric; proteins made up of more than one polypeptide chain, as many of the large ones are, are called oligomeric. Based upon chemical composition, proteins are divided into two major classes: simple proteins, which are composed of only amino acids, and conjugated proteins, which are composed of amino acids and additional organic and inorganic groupings, certain of which are called prosthetic groups. Conjugated proteins include glycoproteins, which contain carbohydrates; lipoproteins, which contain lipids; and nucleoproteins, which contain nucleic acids.
Classified by biological function, proteins include the enzymes, which are responsible for catalyzing the thousands of chemical reactions of the living cell; keratin, elastin, and collagen, which are important types of structural, or support, proteins; hemoglobin and other gas transport proteins; ovalbumin, casein, and other nutrient molecules; antibodies, which are molecules of the immune system (see immunity); protein hormones, which regulate metabolism; and proteins that perform mechanical work, such as actin and myosin, the contractile muscle proteins.
Protein Structure
Every protein molecule has a characteristic three-dimensional shape, or conformation. Fibrous proteins, such as collagen and keratin, consist of polypeptide chains arranged in roughly parallel fashion along a single linear axis, thus forming tough, usually water-insoluble, fibers or sheets. Globular proteins, e.g., many of the known enzymes, show a tightly folded structural geometry approximating the shape of an ellipsoid or sphere.
Because the physiological activity of most proteins is closely linked to their three-dimensional architecture, specific terms are used to refer to different aspects of protein structure. The term primary structure denotes the precise linear sequence of amino acids that constitutes the polypeptide chain of the protein molecule. Automated techniques for amino-acid sequencing have made possible the determination of the primary structure of hundreds of proteins.
The physical interaction of sequential amino-acid subunits results in a so-called secondary structure, which often can either be a twisting of the polypeptide chain approximating a linear helix (α-configuration), or a zigzag pattern (β-configuration). Most globular proteins also undergo extensive folding of the chain into a complex three-dimensional geometry designated as tertiary structure. Many globular protein molecules are easily crystallized and have been examined by X-ray diffraction, a technique that allows the visualization of the precise three-dimensional positioning of atoms in relation to each other in a crystal.
The tertiary structure of several protein molecules has been determined from X-ray diffraction analysis. Two or more polypeptide chains that behave in many ways as a single structural and functional entity are said to exhibit quaternary structure. The separate chains are not linked through covalent chemical bonds but by weak forces of association.
The precise three-dimensional structure of a protein molecule is referred to as its native state and appears, in almost all cases, to be required for proper biological function (especially for the enzymes). If the tertiary or quaternary structure of a protein is altered, e.g., by such physical factors as extremes of temperature, changes in pH, or variations in salt concentration, the protein is said to be denatured; it usually exhibits reduction or loss of biological activity.
Protein Synthesis
The cell's ability to synthesize protein is, in essence, the expression of its genetic makeup. Protein synthesis is a sequence of chemical reactions that occur in four distinct stages, i.e., activation of the amino acids that ultimately will be joined together by peptide bonds; initiation of the polypeptide chain at a cell organelle known as the ribosome; elongation of the polypeptide by stepwise addition of single amino acids to the chain; and termination of amino-acid additions and release of the completed protein from the ribosome. The information for the synthesis of specific amino-acid sequences is carried by a nucleic acid molecule called messenger RNA (see nucleic acid). Proteins are needed in the diet mainly for their amino acids, which the body uses to build new proteins (see nutrition).
The mechanism of action of many widely used antibiotics, such as streptomycin, chloramphenicol, and tetracycline, can be understood in terms of their ability to interfere with some stage of protein synthesis in bacteria.
Protein
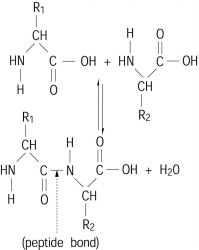
A biological macromolecule made up of various α-amino acids that are joined by peptide bonds. A peptide bond is an amide bond formed by the reaction of an α-amino group (—NH2) of one amino acid with the carboxyl group (—COOH) of another, as shown below. Proteins generally contain from 50 to 1000 amino acid residues per polypeptide chain. See Peptide
Occurrence
Proteins are of importance in all biological systems, playing a wide variety of structural and functional roles. They form the primary organic basis of structures such as hair, tendons, muscle, skin, and cartilage. All of the enzymes, the catalysts in biochemical transformations, are protein in nature. Many hormones, such as insulin and growth hormone, are proteins. The substances responsible for oxygen and electron transport (hemoglobin and the cytochromes, respectively) are conjugated proteins that contain a metalloporphyrin as the prosthetic group. Chromosomes are highly complex nucleoproteins, that is, proteins conjugated with nucleic acid. Viruses are also nucleoprotein in nature. Of the more than 200 amino acids that have been discovered either in the free state or in small peptides, only 20 amino acids are present in mammalian
proteins. Thus, proteins play a fundamental role in the processes of life. See Amino acidsSpecificity
The linear arrangement of the amino acid residues in a protein is termed its sequence (primary structure). The sequence in which the different amino acids are linked in any given protein is highly specific and characteristic for that particular protein.
This specificity of sequence is one of the most remarkable aspects of protein chemistry. The number of possible permutations of sequence in even so small a protein as insulin, of molecular weight 5732 and with 51 amino acid residues, is astronomic: 1051 permutations. Yet it has been established that the pancreatic cell of a given species has only one of these possible sequences. The elucidation of the mechanism conferring such a high degree of specificity on the biosynthetic reactions by which proteins are built up from free amino acids has been one of the key problems of modern biochemistry. See Molecular biology
Proteins are not stretched polymers; rather, the polypeptide backbone of the molecule can fold in several ways by means of hydrogen bonds between the carbonyl oxygen and the amide nitrogen. The folding of each protein is determined by its particular sequence of amino acids. The long polypeptide chains of proteins, particularly those of the fibrous proteins, are held together in a rather well-defined configuration. The backbone is coiled in a regular fashion, forming an extended helix. As a result of this coiling, peptide bonds separated from one another by several amino acid residues are brought into close spatial approximation. The stability of the helical configuration can be attributed to hydrogen bonds between these peptide bonds.
In addition to hydrogen bonds, there are electrostatic interactions, such as those between COO- and NH3+ groups of the side chains, and van der Waals forces, that is, hydrophobic interactions, which help to determine the configuration of the polypeptide chain. The term secondary structure is used to refer to all those structural features of the polypeptide chain determined by noncovalent bonding interactions.
In addition to the α-helical sections of proteins, there are segments that contain β-structures in which there are hydrogen bonds between two polypeptide chains that run in parallel or antiparallel fashion.
The tertiary structure (third level of folding) of a protein comes about through various interactions between different parts of the molecule. Disulfide bridges formed between cysteine residues at different locations in the molecule can stabilize parts of a three-dimensional structure by introducing a primary valence bond as a cross-link. Hydrogen bonds between different segments of the protein, hydrophobic bonds between nonpolar side chains of amino acids such a phenylalanine and leucine, and salt bridges such as those between positively charged lysyl side chains and negatively charged aspartyl side chains all contribute to the individual tertiary structure of a protein.
Finally, for those proteins that contain more than one polypeptide chain per molecule, there is usually a high degree of interaction between each subunit, for example, between the α- and β-polypeptide chains of hemoglobin. This feature of the protein structure is termed its quarternary structure.
Properties
The properties of proteins are determined in part by their amino acid composition. As macromolecules that contain many side chains that can be protonated and unprotonated depending upon the pH of the medium, proteins are excellent buffers. The fact that the pH of blood varies only very slightly in spite of the numerous metabolic processes in which it participates is due to the very large buffering capacity of the blood proteins.
Biosynthesis
The processes by which proteins are synthesized biologically have become one of the central themes of molecular biology. The sequence of amino acid residues in a protein is controlled by the sequence of the DNA as expressed in messenger RNA at ribosomes. See Deoxyribonucleic acid (DNA), Ribonucleic acid (RNA), Ribosomes
Degradation
As with many other macromolecular components of the organism, most body proteins are in a dynamic state of synthesis and degradation (proteolysis). During proteolysis, the peptide bond that links the amino acids to each other is hydrolyzed, and free amino acids are released. The process is carried out by a diverse group of enzymes called proteases. During proteolysis, the energy invested in generation of the proteins is released. See Enzyme
Distinct proteolytic mechanisms serve different physiological requirements. Proteins can be divided into extracellular and intracellular, and the two groups are degraded by two distinct mechanisms. Extracellular proteins such as the plasma immunoglobulins and albumin are degraded in a process known as receptor-mediated endocytosis. Ubiquitin-mediated proteolysis of a variety of cellular proteins plays an important role in many basic cellular processes such as the regulation of cell cycle and division, differentiation, and development; DNA repair; regulation of the immune and inflammatory responses; and biogenesis of organelles.
Molecular chaperones
Molecular chaperones are specialized cellular proteins that bind nonnative forms of other proteins and assist them to reach a functional conformation. The role of chaperone proteins under conditions of stress, such as heat shock, is to protect proteins by binding to misfolded conformations when they are just starting to form, preventing aggregation; then, following return of normal conditions, they allow refolding to occur. Chaperones also play essential roles in folding under normal conditions, providing kinetic assistance to the folding process, and thus improving the overall rate and extent of productive folding.
Protein engineering
The amino acid sequences, sizes, and three-dimensional conformations of protein molecules can be manipulated by protein engineering, in which the basic techniques of genetic engineering are used to alter the genes that encode proteins. These manipulations are used to generate proteins with novel activities or properties for specific applications, to discover structure-function relationships, and to generate biologically active minimalist proteins (containing only those sequences necessary for biological activity) that are smaller than their naturally occurring counterparts.
Many subtle variations in a particular protein can be generated by making amino acid replacements at specific positions in the polypeptide sequence. For example, at any specific position an amino acid can be replaced by another to generate a mutant protein that may have different characteristics by virtue of the single replaced amino acid. Amino acids can also be deleted from a protein sequence, either individually or in groups. These proteins are referred to as deletion mutants. Deletion mutants may or may not be missing one or more functions or properties of the full, naturally occurring protein. Moreover, part or all of a protein sequence can be joined or fused to that of another protein. The resulting protein is called a hybrid or fusion protein, which generally has characteristics that combine those of each of the joined partners.