Many peoples are oblivious to the fact that when they consume certain delicious food products like yogurt, buttermilk or cheese, they are actually eating live bacterial biomass which has acidified the milk content and contains a mixture of bacterial slime layers. Perhaps it is a blessing that most people are unaware because when most hear the word ‘bacteria,’ their first thought is of a microscopic unicellular organism that causes various types of diseases especially if it is found in food. This may be true for most micro organisms, but there are some bacterial species that are essential in the manufacture of those tasty foods, (Johnson-Green, 2002). One such group of bacteria is the Lactic Acid Bacteria (LAB). Kun (2003) has found that it has become increasingly difficult to define this set of organisms as most opinions differ as to which organisms should be a part of this group. However, they can be broadly defined as a large group of related bacteria that have similar properties and convert carbohydrates to lactic acid by lactic acid fermentation.

They are heterotrophic and can be found extensively in nature including plant leaves (e.g. Lactobacillus, Leuconostoc), the animal’s oral cavity (e.g. Streptococcus mutans), digestive system (e.g. Enterococcus faecalis), genitourinary tract (e.g. Lactobacillus) as well as faecal matter, compost and rotting vegetation. These areas normally have an adequate supply of amino acids and vitamins (Murray et al, 1998; internet 1). They are aero tolerant anaerobes which mean they can tolerate oxygen but do not use it, (Johnson- Green, 2002).
The group consist of a number of gram positive bacteria which include the genera; Aerococcus, Bifidobacterium, Carnobacterium, Enterococcus, Lactococcus, Lactobacillus, Lactosphaera, Leuconostoc, Microbacterium, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus and Weissella, (Jay, 2000). They can be categorized into two groups based on the amount of lactic acid produced as the end product. Those that produce lactic acid as its only or major end product using the glycolitic pathway are termed Homolactics. Lactobacillus acidophilus, Lactobacillus bulgarius, Lactobacillus delbrueckii, Lactobacillus helveticus, Streptococcus thermophilus and Enterococcus faecium normally use this homolactic fermentation process, (Kun 2003).
These homolactics are able to produce twice as much energy then heterolactics as homolactics produce two lactic acid molecules from one glucose molecule, (Jay, 2000). Heterolactics produce only one molecule of lactic acid along with carbon dioxide and ethanol or acetate as its major products, (Nester et al, 1998). The bacteria will switch between producing ethanol or acetate depending on its growing environment.
The heterolactic fermentation process is normally used by Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium thermophilum, Lactobacillus fermentum, Lactobacillus salivarius, Lactobacillus casei, Lactobacillus rhamnosus and Lactobacillus plantarum, (Kun, 2003). Jay (2000) has stated that the genus Lactobacillus can be further assembled into three groups based on these fermentation processes. These groups are obligate homofermentative species which only use the glycolitic pathway, facultative heterofermentative species which use the glycolitic pathway but are able to use the heterolactic fermentation process and obligate heterofermentative which only use heterolactic fermentation process.
The levels of optical isomeric forms of lactic acid that are produced are dependant on the bacterial species; these are l-lactic acid produced from Lactococcus and Carnobacterium or d-lactic acid produced from Leuconostoc, (Liu, 2003). The figure illustrates.
D(-) levorotatory lactic acid L(+) dextrorotatory lactic acid
Not copy-writed; Adapted internet 2
L(+) dextrorotatory lactic acid is normally completely metabolized by glycogen synthesis in humans and is rapidly absorbed from the intestine, whereas D(-) levorotatory lactic acid is metabolized at a slower rate. The problem with acid that has not been broken down is that it causes metabolic acidosis in infants, which is why L. sporogenes is preferred as a probiotic in therapeutic formulations as it only produces L(+)- lactic acid, (internet 2).
There are a number of reasons why (LAB) are used in the food industry. The main reason being probiotics, which is consuming food products that contain microbial cells that are beneficial to the human health (Tannock et al. 2000). These bacteria are able to provide some amount of protection from certain pathogens like Clostridium difficile by colonizing the human intestine. It is believed that when Lactobacillus rhamnosus GG and Bifidobacterium spp are used as starter cultures, they are the most effective in guarding against these pathogens. Lactobacillus acidophilus and Lactobacillus bulgaricus are believed to have probiotic possibilities on the immune system, (Johnson-Green, 2002).
These bacteria are normally inoculated into the food material as starter cultures, (Roller and Harlander, 1998). Meat products like salami and chorizo depend on lactic acid fermentation in combination with drying and curing salts to keep their quality, safety and colour. The flavours of these products are contributed by yeast and moulds. The flavours in certain wines are achieved by the break down of malic acid in fruit juice to lactic acid and carbon dioxide. Baker’s yeast and lactic acid bacteria work alongside each other in certain types of bread (e.g. French bread) to improve the structure, flavour and storage life. Vegetables like olives, sauerkraut and gherkins are also prepared by lactic acid fermentation. Various types of LAB are involved along with certain yeast and moulds, (internet 3).
LAB are mainly used in the dairy industry. Dairy starters can be divided into mesophilic cultures; having an optimal temperature of 26 C which are mainly used for production of Cheddar and Gouda cheese, buttermilk and sour cream or thermophilic cultures of optimal temperatures of 42 C, mainly used for the production of yogurt and high scald cheeses. Members of the mesophilic group belong to the Lactococcus lactis subspp, while members of the thermophilic group are Streptococcus thermophilus and some members of the Lactobacillus species, (Roller and Harlander, 1998).
The process of milk fermentation is done by the inoculation of starter cultures of LAB. This lowers the pH by the fermentation of lactose (which is the major sugar in milk) due to the process of the lactic acid production, (Nester et al, 1998). This decrease in pH allows the foods to have a prolonged self life because it can fall to as low as 4.0 which inhibits the growth of many acid sensitive bacteria (including the most common human pathogens) while the LAB are acid tolerant and continue growing (Nester et al, 1998; internet 1). Only the genus Pediococcus causes deterioration of food which results in food spoilage, but generally the LAB are not pathogenic to humans and do not cause diseases.
To make yogurt the pH of milk has to be lowered enough (normally using Streptococcus thermophilus and Lactobacillus bulgaricus as a mixed starter culture) to cause the casein in it to start coagulating which creates a semisolid product. If this is incubated at about 40 C, then the texture of the milk changes as a result of the coagulating milk proteins (curd). Under controlled conditions only organisms that produce desired end products will grow, (Nester et al, 1998).
Cheese is also made from milk with the participation of lactic acid bacteria. The enzyme that converts soluble caseinogen to insoluble protein is called rennin. It had been extracted from the stomach of calves in previous years, but due to problems encountered like unstable supply due to global demand and differences in purity and activity from different suppliers, more recent techniques have allowed it to be made from genetically engineered organisms, (Nester et al, 1998). If this enzyme is added to the semisolid material at a crucial level of lactic acid production, then this results in the coagulated protein or the curd being quite firm and is developed into cheese after being shrunk, pressed, salted and ripened. The liquid portion called the whey is drained or pressed from the curd, (Johnson-Green, 2002). Various different types of cheese can be manufactured depending on how it is allowed to ripen. Example if Penicillum caseicolum is added to the surface of the cheese wheel, then the mycelia will produce enzymes that alter the texture and flavour producing cheese known as Brie or Camembert. Similarly Limburger can be made with the addition of the bacterium Brevibacterium linens, (Nester et al, 1998).
Different industrial companies have their different techniques of producing lactic acid, however based on research done by Büyükkileci and Harsa (2004), optimal productivity of lactic acid is produced from Lactobacillus casei at a temperature of 37 C and pH 5.5. Productivity reached as high as 3.97g dm-3 h-1 in their batch fermentation process. Product yields were roughly 0.93 grams of lactic acid per gram of lactose. However, using micro or ultrafiltration to continuously separate products from bacteria greatly enhances the productivity, (Moueddeb et al, 1995). The reason for this is lactic acid that has been produced has a toxic effect on the bacteria, thus lowering productivity, (Luedeking and Piret, 1959). Although this method has improved yield, there is a major problem that still exist with the processing of milk products; i.e. the disposal of the whey after separation from the useful properties. World production normally amounts to about eighty-two million metric tons. Due to its high chemical oxygen demand it is very expensive to discard. It contains inorganic salts and lactose as its main component, but this is of little significance as the inorganic salts are not very useful and lactose is a very poor sweetener. But, since lactose can be converted to lactic acid for use in the food and chemical industries, Professor Herwig Brunner of the University of Stuttgart Germany has identified an environmentally friendly and low-cost technique of producing lactic acid from whey so as to combine the manufacturing of valuable materials with lowering the amount of the organic waste discarded.
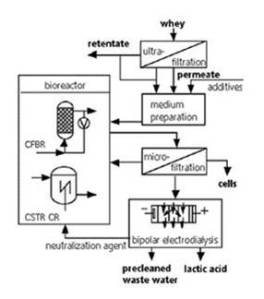
The system firstly involves the separation of the curd from the whey. The remaining whey is sent to an unaerated bioreactor where the lactose content is converted to lactic acid by LAB. A cross-flow-filtration unit is used to separate the lactic acid from the biomass and high productivities are accomplished by cell recycling. A process known as bipolar electrodialysis is done for product recovery (downstream processing). In is process the crude solution is separated from the pure lactic acid solution using ion selective membranes in an electric field, (internet 4).
Key
CSTR CR: continuously operated stirred tank reactor with cell recycling;
CFBR: continuously operated fluidized-bed reactor.
The figure illustrates.
Not-copywrited; Adapted from internet 4
The major advantage with this method is it reduces the cost of disposing unwanted whey substances by converting lactose that would have normally been discarded to lactic acid that can be used in the food industry or the chemical industry, (internet 4). However, this process has only been tested in the laboratory. Scale–up calculations estimates only four thousand two hundred tons of lactic acid being produced from one hundred thousand tons of acid whey. So although the process helps in disposal and decreases the cost of a kg of lactic acid from 1.20 to 0.80 Euro, there is still a large quantity of waste material left. There is also the increased cost of obtaining specialized equipment and technical staff.
Another problem with the processing of fermented milk is starter failure caused by bacteriophage. A bacteriophage is a virus that specifically infects bacteria where it undergoes replication and destroys the host cell before spreading to other bacteria, (Brown, 2001). These bacteriophage can spread throughout the fermentation vat very quickly, thereby stopping production of lactic acid and leaving the milk susceptible to spoilage especially to the pathogen Staphylococcus aureus which can easily cause an outbreak by an accretion of its toxins. Most bacteriophage are unaffected by pasteurization because they are heat resistant, hence one solution to this problem is to further heat the milk to 900C for about thirty minutes as it is believed that many bacteriophage cannot survive this temperature, (Johnson-Green, 2002), however this temperature may destroy some components of the milk (e.g. denature protein) as well as increase the power consumption thereby increasing cost. Other solutions include using phage-resistant strains of LAB which have been genetically modified to produce restriction enzymes that degrade the virus DNA. This could cause a problem in the long-run as there are always ethical issues surrounding genetically engineered bacteria because they can mutate which will cause further problems. A third solution is the use of phage-inhibitory media to grow starter cultures. This can be done by adding calcium chelators to the medium as bacteriophages need calcium to multiply. The disadvantage with this method is the growth of the LAB is also hampered, (Johnson-Green, 2002).
The dominance of the LAB over other organisms is not only based on its ability to reduce pH but they can also produce secondary metabolites (antibiotics) that kill other bacteria. Bacteriocins are the most important group and be categorized as antimicrobial peptides, proteins or protein complexes, (Demain and Davis, 1999). The first bacteriocin was discovered in 1947 and was called nisin. Nisin A is produced from Lactococcus lactis sub sp. Lactis and is widely used in the production of cheese, (Kun, 2003). Since its discovery, there has been a lot a research done on this bacteriocin. One such study involves the improvement of oral hygiene. Howell et al (1993) was successful in showing that nisin is effective against plague build-up and gingivitis in beagle dogs. Dr. Hillman of the University of Florida has gone a step further and has targeted the bacteria which are the major cause of tooth decay, (internet 5). There are hundreds of bacteria in the oral cavity but researchers has identified that lactic acid fermentation caused by the bacterium Streptococcus mutans is the major cause of tooth decay as it lives on sugar and produces the lactic acid which breaks down the enamel of the tooth, (Pollack, 2004; internet 5). Dr. Williams genetically modified S. mutans by removing the gene for producing lactic acid and replacing it with a gene that produces alcohol which allows the bacterium to get rid of its waste. The idea is to replace the normal S. mutans with the genetically modified version by allowing them to produce an antibiotic that kills normal S. mutans.
The success story is seen in laboratory mice fed with a high sugar diet where the GM bacteria reduced cavities by half compared to the control. However, there are still some uncertainties about this method. The antibiotic produced by the GM bacteria may be able to kill the normal flora of the mouth, which will cause the delicate balance to be shifted; the GM bacteria may be able to be spread to others by kissing; (internet 5) and may also be able to mutate in the long term causing more harm than good.
Other research carried out in the laboratory on GM LAB; involve the development of efficient mucosal vaccines for delivery of protective antigens to the blood stream by live LAB vectors. It has been stated that when the antigen was administered to mice using L. lactis, S. gordonii and Lactobacillus spp., the systemic and mucosal antigen-specific immune responses were elicited, (Mercenier, 2000).
LAB is beneficial to persons who are lactose intolerant and cannot digest certain dairy products like milk or ice cream because they have reduced amounts or absence of the intestinal enzyme lactase. This allows bacteria in the colon to utilize the lactose that has not been absorbed producing increased levels of hydrogen gas and hence diarrhoea, (Jay, 2000). However studies done by Alm (1982) has shown that when mice which are lactose intolerant are given products containing LAB, they had fewer symptoms depicting lactose intolerance compared to the control. This is because the lactic acid bacteria supplied the enzymes required to break down lactose.
LAB does not only keep the gastrointestinal tract healthy, but according to Professor Hisakazu Lino of the Showa Women’s University, it has proven to be effective against pollen allergy symptoms. Research done on allergic patients has shown that the production of interleukin-2 (IL-2) and interferon- (IFN-) by TH1 cells induce macrophage activation which helps to destroy intracellular pathogens. TH2 cells on the other hand secrete IL-4, IL-5 and IL-13 which help B-cells in producing antibodies (e.g. IgE) to eliminate extracellular pathogens, (Biedermann et al, 2004). It is necessary to have a balance between TH1 and TH2 cells to maintain a non-allergenic life. The overproduction of IgE is what initiates pollen allergy symptoms. Professor Lino and his team with help from Kirin Brewery Co.’s Central Laboratories for Key Technology have shown that the level of IgE antibodies in mice with pollen allergies has decreased by as much as fifty percent as a result of the injection of KW lactic acid bacteria. It was also noted that the TH1 activity increased by about six times in the fourteen week experiment, (Internet 6). Professor Lino has not stated the corrective action to be taken if the balance was tilted in the other direction i.e. if the KW lactic acid bacteria caused too much TH1 to be produced, as this would cause another problem.
LAB have been beneficial in human for many years, but some of species do cause human diseases as there have been sixty eight reports of human clinical illness over a 50 year period. The third leading cause of hospital acquired (nosocomial) infections is the Enterococci, with E. faecium and E. faecalis being the major species. There have also been eighteen reports of Pediococci infections over a three-year period and twenty-seven reports of Leuconostocs infections over a period of seven years. Data analyzed by Aquirre and Collins (1993) have suggested that LAB cause opportunistic infections and are not normally capable of harming healthy humans.
LAB has been used in food industries as a probiotic and means of improving shelf life and flavour for a number of food products. With the advance in technology, researchers are finding new and improved ways (e.g. genetic manipulation) of using its antimicrobial and other properties. For every positive result obtained in research, there is at least one problem, but this can be an advantage in itself as scientist would be working to correct that problem for the betterment of the product.
Reference
Aguirre, M., and Collins, M. D. 1993. Lactic acid bacteria and human clinical infection. Journal of Applied Bacteriology. 75: 95-107
Alm, L. 1982. Effects of fermentation of lactose, glucose and galactose content in milk and suitability of fermented milk products for lactose intolerant individuals. Journal of Dairy Science. 65: 346-352.
Biedermann, T., Röcken, M., Carballido, J. (2004) TH1 and TH2 Lymphocyte Development and Regulation of TH Cell Mediated Immune Responses of the Skin. Journal of Investigative Dermatology Symposium Proceedings 9 Issue 1 pg 5
Brown, T.A. (2001). Gene Cloning and DNA Analysis (4th Edition). Blackwell Science, Manchester.
Büyükkileci, A. and Harsa, S. (2004). Batch production of L(+) lactic acid from whey by Lactobacillus casei (NRRL B-441). Journal of Chemical Technology & Biotechnology, 79, no. 9, pp. 1036-1040
Demain, A., L. and Davies, J., E. (ed) 1999. Manual of Industrial Microbiology and Biotechnology (2nd edition). ASM Press, Washington, D.C.
Howell, T.H., Fiorellini, J.P., Blackburn, P., Projan, S.J., de la Harpe, J. and Williams, R.C. 1993. The effect of a mouthwash based on nisin, a bacteriocin, on developing plague and gingivitis in beagle dogs. J. Clin. Periodontol. 20: 335-339.
Jay, J. (2000) Modern Food Microbiology (6th edition). Aspen, Maryland
Johnson-Green, P. (2002) Introduction to Food Biotechnology CRC Press, Boca Raton.
Kun, L.Y. (ed). (2003) Microbial Biotechnology-Principles and Applications. World Scientific, USA.
Liu, S.Q. (2003) Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. International Journal of Food Microbiology 83, 115131
Luedeking R. and Piret E.L.. 1959. A kinetic study of lactic acid fermentation batch process at controlled pH. J. Biochem. Microbiol. Technol. Eng., 4 393-412
Mercenier A, Muller-Alouf H, Grangette C. 2000. Lactic acid bacteria as live vaccines. Curr Issues in Mol Biol. 2(1):17-25
Murray, P., Rosenthal, K., Kobayashi, G., Pfaller, M. (1998) Medical Microbiology (3rd edition) Mosby, Missouri.
Roller, S. and Harlander, S (ed) 1998. Genetic Modification in the Food Industry. Blackie Academic & Professional
Tannock, G.W., Munro, K., Harmsen, H.J., Welling, G.W., Smart, J. and Gopal, P.K. (2000) Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Applied and Environmental Microbiology 66, 25782588.
Internet References
Internet 1
Dugas, J
Lactic Acid Bacteria
On the web site of Waksman Foundation for Microbiology
http://www.waksmanfoundation.org/labs/mbl/lactic.html
Internet 2
No author given
Lactospore
Sabinsa Corporation
http://www.lactospore.com/back.htm
Internet 3
No Author Given
Lactic acid fermentations
http://www.schoolscience.co.uk/content/4/biology/sgm/sgmprods2.html
Internet 4
Professor Herwig Brunner
Institute for Interfacial Engineering
University of Stuttgart Germany
http://www.uni-stuttgart.de/igvt/index.en.html
Internet 5
Andrew Pollack (2004)
Bacteria Enlisted for New Trials on Dental Health
On the website of The New York Times
http://www.nytimes.com/2004/11/30/health/30tooth.html
Internet 6
Prof. Hisakazu Iino
Showa Women’s University
On the web site of The Yomiuri Shimbun
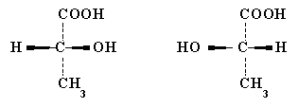

It’s actually a cool and useful piece of information.
I am happy that you shared this helpful info with us. Please stay us up to date like this.
Thank you for sharing.