Abstract
Main conclusion
Five promoters of the cold-inducible rice genes were isolated. The quantitative and qualitative expression analyses in the high generation transgenic rice suggest that the genes are stably induced by low temperature.
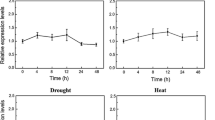
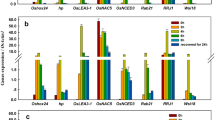
Cold-inducible promoters are highly desirable for stress-inducible gene expression in crop genetic engineering. In this study, five rice genes, including OsABA8ox1, OsMYB1R35, OsERF104, OsCYP19-4, and OsABCB5, were found to be transcriptionally induced by cold stress. The promoters of these five genes were isolated, and their activities were identified in various tissues of transgenic rice plants at different growth stages both before and after cold stress. Histochemical staining, quantitative fluorescence assays, and GUSplus gene expression assays in corresponding promoter-GUSplus transgenic rice plants confirmed that the five promoters were cold-inducible with different expression patterns and strengths. The OsABA8ox1 and OsERF104 promoters had very low background expression; in contrast, the OsMYB1R35 promoter had higher basal activity in the roots, and OsCYP19-4 promoter activity was preferentially high in leaves and flowers of untreated transgenic lines. The OsABCB5 promoter had the highest basal activity among the five promoters. After cold induction, the activities of the OsABA8ox1, OsMYB1R35, and OsABCB5 promoters were high in both roots and leaves, slightly lower than that of the constitutively expressed OsActin1 promoter but comparable to that of the AtRD29A promoter. During the cold treatment time course, the activities of OsABA8ox1 and OsABCB5 promoters were quickly up-regulated in the early period and peaked at 24 h, after which the induction level gradually decreased until 48 h. The activities of the OsMYB1R35 and OsCYP19-4 promoters increased under stress in a time-dependent manner, while OsERF104 promoter activity began to increase at 4 h and then decreased strongly. Furthermore, activities’ analysis in T3, T4, and T5 homozygous progeny of single-copy plants revealed that five promoters maintained their activities at comparable levels with no evidence of silencing under cold stress. Overall, the five cold-inducible rice promoters described herein could potentially be used in crop biotechnology.






Similar content being viewed by others
References
Baker SS, Wilhelm KS, Thomashow MF (1994) The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol 24(5):701–713
Barry GF, Rogers SG, Hein MB, Niedermeyer JG, Hoffman NL, Blatt LM, Fraley RT, Sharp CR, Horsch RB (1985) Identification of cytokinin genes and transfer into plants. In: Current topics in plant biochemistry and physiology, vol 4. Proceedings of the plant biochemistry and physiology symposium, Columbia, pp 101–109
Belintani NG, Guerzoni JTS, Moreira RMP, Vieira LGE (2012) Improving low-temperature tolerance in sugarcane by expressing the ipt gene under a cold inducible promoter. Biol Plant 56(1):71–77
Chai C, Subudhi PK (2016) Comprehensive analysis and expression profiling of the OsLAX and OsABCB auxin transporter gene families in rice (Oryza sativa) under phytohormone stimuli and abiotic stresses. Front Plant Sci 7:593
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12(10):444–451
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18(4):675–689
Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23(3):567–581
Czaja W, Miller KY, Skinner MK, Miller BL (2014) Structural and functional conservation of fungal MatA and human SRY sex-determining proteins. Nat Commun 5(5):5434
Dai X, Chong K (2010) Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Pediatr Ann 39(11):709–713
Datta K, Baisakh N, Ganguly M, Krishnan S, Yamaguchi Shinozaki K, Datta SK (2012) Overexpression of Arabidopsis and rice stress genes’ inducible transcription factor confers drought and salinity tolerance to rice. Plant Biotechnol J 10(5):579–586
Duan Y, Zhai C, Li H, Li J, Mei W, Gui H, Ni D, Song F, Li L, Zhang W, Yang J (2012) An efficient and high-throughput protocol for Agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in Japonica rice (Oryza sativa L.). Plant Cell Rep 31(9):1611–1624. doi:10.1007/s00299-012-1275-3
Dunn MA, White AJ, Vural S, Hughes MA (1998) Identification of promoter elements in a low-temperature-responsive gene (blt4.9) from barley (Hordeum vulgare L.). Plant Mol Biol 38(4):551–564
Gutha L, Reddy A (2008) Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant Mol Biol 68(6):533–555
Holme IB, Wendt T, Holm PB (2013) Intragenesis and cisgenesis as alternatives to transgenic crop development. Plant Biotechnol J 11(4):395–407
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47(1):141–153. doi:10.1093/pcp/pci230
Jang IC, Choi WB, Lee KH, Song SI, Nahm BH, Kim JK (2002) High-level and ubiquitous expression of the rice cytochrome c gene OsCc1 and its promoter activity in transgenic plants provides a useful promoter for transgenesis of monocots. Plant Physiol 129(4):1473–1481. doi:10.1104/pp.002261
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13):3901–3907
Jiang C, Iu B, Singh J (1996) Requirement of a CCGAC cis-acting element for cold induction of the BN115 gene from winter Brassica napus. Plant Mol Biol 30(3):679–684
Jiang Y, Peng D, Bai LP, Ma H, Chen LJ, Zhao MH, Xu ZJ, Guo ZF (2013) Molecular switch for cold acclimation—anatomy of the cold-inducible promoter in plants. Biochemistry 78(4):342–354
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45(3):346–350
Khodakovskaya M, Li Y, Li J, Vaňková R, Malbeck J, Mcavoy R (2005) Effects of cor15a-IPT gene expression on leaf senescence in transgenic Petunia × hybrida and Dendranthema × grandiflorum. J Exp Bot 56(414):1165–1175
Kim SH, Choi HS, Cho YC, Kim SR (2011) Cold-responsive regulation of a flower-preferential class III peroxidase gene, OsPOX1, in rice (Oryza sativa L.). J Plant Biol 55(2):123–131
Kovalchuk N, Wei J, Eini O, Morran S, Pyvovarenko T, Fletcher S, Bazanova N, Harris J, Beck-Oldach K, Shavrukov Y (2013) Optimization of TaDREB3 gene expression in transgenic barley using cold-inducible promoters. Plant Biotechnol J 11(6):659–670
Lafitte HR, Ismail A, Bennett J (2004) Abiotic stress tolerance in rice for Asia: progress and the future. In: Fischer T, Turner N, Angus J, McIntyre L, Robertson M, Borrell A (eds) New directions for a diverse planet. Proceedings of the fourth international crop science congress, Brisbane, 26 Sep–1 Oct 2004
Li Y, Hagen G, Guilfoyle TJ (1992) Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol 153(2):386–395
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, Arabidopsis. Plant Cell 10(8):1391–1406
Liu K, Wang L, Xu Y, Chen N, Ma Q, Li F, Chong K (2007) Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta 226(4):1007–1016
Lu J, Sivamani E, Li X, Qu R (2008) Activity of the 5′ regulatory regions of the rice polyubiquitin rubi3 gene in transgenic rice plants as analyzed by both GUS and GFP reporter genes. Plant Cell Rep 27(10):1587–1600
Mcelroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2(2):163–171
McElroy D, Blowers A, Jenes B, Wu R (1991) Construction of expression vectors based on the rice actin 1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet 231(1):150–160. doi:10.1007/bf00293832
Mega R, Meguromaoka A, Endo A, Shimosaka E, Murayama S, Nambara E, Seo M, Kanno Y, Abrams SR, Sato Y (2015) Sustained low abscisic acid levels increase seedling vigor under cold stress in rice (Oryza sativa L.). Sci Rep 5:13819
Meng L, Song B, Zhang Q, Xun L, Yuan L, Ou Y, Zhang H, Liu J (2013) A synthetic tuber-specific and cold-induced promoter is applicable in controlling potato cold-induced sweetening. Plant Physiol Biochem 67(3):41–47
Mukhopadhyay A, Khush GS (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci USA 101(16):6309–6314
Odell JT, Nagy F, Chua N-H (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313(6005):810–812
Ouellet F, Vazquez-Tello A, Sarhan F (1998) The wheat wcs120 promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Lett 423(3):324–328
Park SH, Yi N, Kim YS, Jeong MH, Bang SW, Yang DC, Kim JK (2010) Analysis of five novel putative constitutive gene promoters in transgenic rice plants. J Exp Bot 61(9):2459–2467
Pegoraro C, Farias DDR, Mertz LM, Santos RSD, Maia LCD, Rombaldi CV, Oliveira ACD (2013) Ethylene response factors gene regulation and expression profiles under different stresses in rice. Theor Exp Plant Physiol 25(4):261–274
Pierre CS, Crossa JL, Bonnett D, Reynolds MP (2012) Phenotyping transgenic wheat for drought resistance. J Exp Bot 63(5):1799–1808
Popa C, Nicola A, Rüde U (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. Vitro Cell Dev Biol Plant 40(1):1–22
Qi Z, Chen Q, Wang S, Hong Y, Wang Z (2014) Rice and cold stress: methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice 7(1):1–12
Sanghera GS, Wani SH, Hussain W, Singh NB (2011) Engineering cold stress tolerance in crop plants. Curr Genom 12(1):30–43
Sarker S, Biswas S, Shahed A, Razzaque S, Seraj ZI (2016) Cloning, characterization and analysis of the Arabidopsis RD29A promoter for its inducible expression in rice under salinity and drought stress. Biores Commun 2(1):139–145
Smita S, Katiyar A, Pandey DM, Chinnusamy V, Bansal KC (2011) Expression network analysis of abiotic stress responsive Myb in rice. In: Biocomp’11—the 2011 international conference on bioinformatics and computational biology, 2011
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94(3):1035–1040
Su CF, Yu SM (2010) A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol 153(1):145–158
Tian Y, Zhang H, Pan X, Chen X, Zhang Z, Lu X, Huang R (2011) Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res 20(4):857–866
Yamaguchi-Shinozaki K, Shinozaki K (1993) Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236(2–3):157–160
Yang L, Ding J, Zhang C, Jia J, Weng H, Liu W, Zhang D (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23(10–11):759–763. doi:10.1007/s00299-004-0881-0
Yang A, Dai X, Zhang WH (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot 63(7):2541–2556
Yoon DH, Sang SL, Park HJ, Lyu JI, Chong WS, Liu JR, Kim BG, Ahn JC, Cho HS (2015) Overexpression of OsCYP19-4 increases tolerance to cold stress and enhances grain yield in rice (Oryza sativa L.). J Exp Bot 67(1):69–82
Zarka DG, Vogel JT, Cook D, Thomashow MF (2003) Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol 133(2):910–918
Zhu J, Dong CH, Zhu JK (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10(3):290–295
Zhu Q, Song B, Zhang C, Ou Y, Xie C, Liu J (2008) Construction and functional characteristics of tuber-specific and cold-inducible chimeric promoters in potato. Plant Cell Rep 27(1):47–55
Acknowledgements
This work was supported by the Genetically Modified Breeding Major Projects (No. 2016ZX08010-002-008), the National Natural Science Foundation of China (Nos. 31401454 and 31501239), the Natural Science Foundation of Anhui Province (No. 1708085QC60), and the Creative Foundation of Anhui Agricultural Academy of Sciences (Nos. 13C0101 and 17A0102).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Qin, R., Xu, R. et al. Isolation and identification of five cold-inducible promoters from Oryza sativa . Planta 247, 99–111 (2018). https://doi.org/10.1007/s00425-017-2765-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2765-x