Abstract
Introduction and hypothesis
In Japan, the authors of urology clinical practice guidelines (UCPG) used in patient-centered care are often targeted by pharmaceutical companies with financial payments. However, the financial relationship between UCPG authors and pharmaceutical companies remains unclear. This study aimed to determine the characteristics of industry payments to physicians that may influence recommendations in UCPG and to assess the transparency of payment disclosure.
Methods
We considered 193 UCPG authors receiving payments from 79 companies between 2016 and 2017 and the 13 UCPG published by the Japanese Urological Association between 2015 and 2018. We determined 2-year combined mean and median payments to authors, total company payments, and associations between author attributes and payment values using multivariate negative binomial regression. Also, we assessed the extent of the financial disclosure policies among the 13 UCPG.
Results
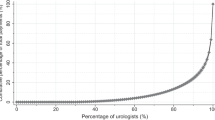
Overall, 171 (88.6%) authors received payments with a combined value of $6,169,333. Median and mean payments were $7147 (interquartile range, $1512–$44,807) and $31,965 (standard deviation, $51,684), respectively. University professors working on multiple UCPG with new drug approvals were associated with higher payments. Twelve (92.3%) UCPG failed to disclose financial conflicts.
Conclusions
While it remains unclear whether financial entanglements improperly influence the contents of UCPG, most Japanese authors received substantial payments from pharmaceutical companies with little or no disclosure. Because insufficient transparency in disclosure of these financial entanglements may compromise the integrity of UCPG, more rigorous regulation and greater disclosure of financial conflicts of interest are needed.

Similar content being viewed by others
Abbreviations
- AUA:
-
American Urological Association
- FCOI:
-
financial conflicts of interest
- GI:
-
Gini index
- IQR:
-
interquartile range
- JPMA:
-
Japan Pharmaceutical Manufacturers Association
- JUA:
-
Japanese Urological Association
- UCPG:
-
Urology Clinical Practice Guidelines
References
Ozieranski P, Rickard E, Mulinari S. Exposing drug industry funding of UK patient organisations. BMJ. 2019;365:l1806. https://doi.org/10.1136/bmj.l1806.
Fabbri A, Gregoraci G, Tedesco D, Ferretti F, Gilardi F, Iemmi D, et al. Conflict of interest between professional medical societies and industry: a cross-sectional study of Italian medical societies’ websites. BMJ Open. 2016;6(6):e011124. https://doi.org/10.1136/bmjopen-2016-011124.
Ozieranski P, Csanadi M, Rickard E, Tchilingirian J, Mulinari S. Analysis of pharmaceutical industry payments to UK health care organizations in 2015. JAMA Netw Open. 2019;2(6):e196253. https://doi.org/10.1001/jamanetworkopen.2019.6253.
Fabbri A, Santos A, Mezinska S, Mulinari S, Mintzes B. Sunshine policies and murky shadows in Europe: disclosure of pharmaceutical industry payments to health professionals in nine European countries. Int J Health Policy Manag. 2018;7(6):504–9. https://doi.org/10.15171/ijhpm.2018.20.
Japan Pharmaceutical Manufacturers Association. Transparency guideline for the relation between corporate activities and medical institutions [in Japanese]; 2018. http://www.jpma.or.jp/english/policies_guidelines/transparency_guideline.html. Accessed 14 Dec 2019.
Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–30. https://doi.org/10.1136/bmj.318.7182.527.
DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med. 2016;176(8):1114–22. https://doi.org/10.1001/jamainternmed.2016.2765.
Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. https://doi.org/10.1136/bmjopen-2017-016408.
Choudhry NK, Stelfox HT, Detsky AS. Relationships between authors of clinical practice guidelines and the pharmaceutical industry. JAMA. 2002;287(5):612–7. https://doi.org/10.1001/jama.287.5.612.
Horn J, Checketts JX, Jawhar O, Vassar M. Evaluation of industry relationships among authors of otolaryngology clinical practice guidelines. JAMA Otolaryngology–Head & Neck Surgery. 2018;144(3):194–201. https://doi.org/10.1001/jamaoto.2017.2741.
Carlisle A, Bowers A, Wayant C, Meyer C, Vassar M. Financial conflicts of interest among authors of urology clinical practice guidelines. Eur Urol. 2018;74(3):348–54. https://doi.org/10.1016/j.eururo.2018.04.023.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Research ZM. Prostate cancer therapeutics market: by therapy (hormone therapy, targeted therapy, chemotherapy, and biologic therapy) and by distribution channel (hospital pharmacies, online sales, retail pharmacies, and others): global industry perspective, comprehensive analysis, and forecast, 2020 – 2026: Zion Marcket Research; 2020.
Foundation for Promotion of Cancer Research. ANCER STATISTICS IN JAPAN ’19. Foundation for Promotion of Cancer Research; 2020. https://ganjoho.jp/data/reg_stat/statistics/brochure/2019/cancer_statistics_2019.pdf. Accessed 24 May 2020.
Oy MT, Gotoh M, Homma Y, Asakura H, Yamanishi T, Yoshida M, et al. Clinical guidelines for overactive bladder syndrome. 2nd ed: RichHill Medical Inc; 2015.
Saito H, Ozaki A, Kobayashi Y, Sawano T, Tanimoto T. Pharmaceutical company payments to executive board members of professional medical associations in Japan. JAMA Intern Med. 2019;179(4):578–80. https://doi.org/10.1001/jamainternmed.2018.7283.
The Japanese Urological Association. The Japanese Urological Association webpage. The Japanese Urological Association; 2020. https://www.urol.or.jp/top.html. Accessed 31 May 2020.
Ozaki A, Saito H, Onoue Y, Sawano T, Shimada Y, Somekawa Y, et al. Pharmaceutical payments to certified oncology specialists in Japan in 2016: a retrospective observational cross-sectional analysis. BMJ Open. 2019;9(9):e028805. https://doi.org/10.1136/bmjopen-2018-028805.
Subramanian S. An elementary interpretation of the Gini inequality index. Theor Decis. 2002;52(4):375–9. https://doi.org/10.1023/A:1020237003687.
American Urological Association. Disclosure of conflicts of interest principles, policies & procedures for managing conflicts of interest. American Urological Association; 2018. https://www.auanet.org/guidelines/disclosure-of-conflicts-of-interest. Accessed 23 May 2020.
Bjartell A, Mottet N, Blok B, De Santis M, Gravas S, Türk C, et al. Managing conflicts of interest in the EAU Guidelines. European Association of Urology; 2017. https://uroweb.org/wp-content/uploads/Managing-Conflict-of-Interest-06072017.pdf. Accessed 23 May 2020.
Institute of Medicine. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011. https://doi.org/10.17226/13058.
Norris SL, Holmer HK, Ogden LA, Burda BU. Conflict of interest in clinical practice guideline development: a systematic review. PLoS One. 2011;6(10):e25153. https://doi.org/10.1371/journal.pone.0025153.
Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–40. https://doi.org/10.1001/jama.2012.156703.
Khan R, Scaffidi MA, Rumman A, Grindal AW, Plener IS, Grover SC. Prevalence of financial conflicts of interest among authors of clinical guidelines related to high-revenue medications. JAMA Intern Med. 2018;178(12):1712–5. https://doi.org/10.1001/jamainternmed.2018.5106.
Saito H, Ozaki A, Sawano T, Shimada Y, Tanimoto T. Evaluation of pharmaceutical company payments and conflict of interest disclosures among oncology clinical practice guideline authors in Japan. JAMA Netw Open. 2019;2(4):e192834. https://doi.org/10.1001/jamanetworkopen.2019.2834.
Sullivan HW, Aikin KJ, Chung-Davies E, Wade M. Prescription drug promotion from 2001-2014: data from the U.S. Food and Drug Administration. PLoS One. 2016;11(5):e0155035. https://doi.org/10.1371/journal.pone.0155035.
Jacob NT. Drug promotion practices: a review. Br J Clin Pharmacol. 2018;84(8):1659–67. https://doi.org/10.1111/bcp.13513.
Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med. 2007;357(7):673–81. https://doi.org/10.1056/NEJMsa070502.
Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, Othman N, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7(10):e1000352. https://doi.org/10.1371/journal.pmed.1000352.
Acknowledgments
Authors thank Dr. Derek Hagman for providing constructive opinion and English language editing.
Funding
This study was funded in part by the Medical Governance Research Institute, a non-profit enterprise that receives donations from pharmaceutical companies, including Ain Pharmaciez, Inc., other organizations, and private individuals. This study also received support from the Waseda Chronicle, an independent non-profit news organization dedicated to investigative journalism. None of the entities providing financial support for this study contributed in any way to the design, execution, data analyses, or interpretation of study findings and the drafting of this manuscript.
Author information
Authors and Affiliations
Contributions
K Yamamoto: Project development, Data collection, Data analysis, and Manuscript writing.
A Murayama: Project development, Data collection, Data analysis, and Manuscript writing.
A Ozaki: Project development, Data collection, Data analysis, and Manuscript writing.
H Saito: Project development, Data collection, and Data analysis.
T Sawano: Project development, Data collection, and Data analysis.
T Tanimoto: Project development, Data collection, Data analysis, and Manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Saito received personal fees from TAIHO Pharmaceutical Co., Ltd., outside the scope of the submitted work. Drs. Ozaki and Tanimoto received personal fees from Medical Network Systems outside the scope of the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamoto, K., Murayama, A., Ozaki, A. et al. Financial conflicts of interest between pharmaceutical companies and the authors of urology clinical practice guidelines in Japan. Int Urogynecol J 32, 443–451 (2021). https://doi.org/10.1007/s00192-020-04547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04547-3