Autism
Gut, Brain, and Microbiome, oh my! Insights into ASD
New research on the immune system, gut bacteria, and their importance to ASD.
Posted July 28, 2018
Ann Marie Martin was my co-author for this post. Ann Marie is a PhD student at UC Riverside in the Special Education program.
A new study in the Journal of Immunology (published July 2nd, 2018), provides important information about the relationship between gut, the immune system, and the developing brain. This study has garnered attention from the media, including IFL Science and Science Daily. Though the article uses mouse models (which we have discussed not being the most accurate models of ASD), I wanted to discuss insights and findings from the article in this post.
First: What did the authors do, and what does it tell us about autism?
To understand what the authors did, we first need to know what their hypotheses were. The authors hypothesized that in mice, ASD-like behaviors might be triggered by an immune response to a molecule called interleukin-17a (IL-17a), which is produced by the immune system. IL-17a is not a newly discovered molecule--it has been associated with rheumatoid arthritis, multiple sclerosis, and psoriasis.
To test their hypothesis, the authors used mice from two separate laboratories, known as Jax and Tac. The Tac mice had microflora in their gut which made them prone to an IL-17a inflammatory response. The Jax mice did not have that specific microflora in their gut.
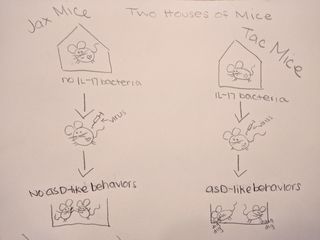
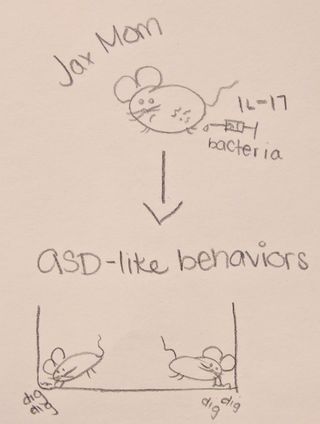
First, both sets of mice were exposed to a virus designed to produce an immune response (and thus IL-17a). Researchers found that Tac mice, who had gut bacteria making them prone to an inflammatory response, had babies (called pups) who developed ASD-like behaviors (e.g. less social interest, more repetitive digging and burying behaviors). The Jax mice, without the gut bacteria, had pups who did not display ASD-like behaviors. This first finding is interesting because it suggests there is an important interaction between the micro biome (gut) and immune response in mothers which affects neural development of babies. In these mouse models, this relationship appears to confer specific risk for the pup developing ASD-like behaviors.
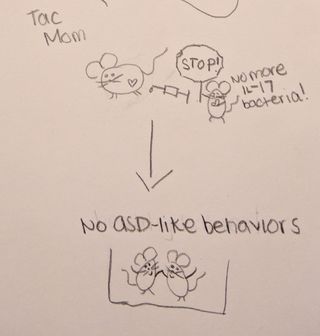
Then, the researchers wanted to explore whether the immune-response was a necessary component of the mouse offspring developing ASD-like behaviors. The researchers artificially blocked the IL-17a molecule (which would prevent the inflammatory response). When IL-17a was blocked, the pups from the Tac mice were born without ASD-like behaviors. This finding suggests that when the immune-system response was blocked, the microbiome of the mother did not confer specific risk for ASD in this model. This provides further evidence that the relationship between gut and immune response is important for the development of ASD-like behaviors in mice.
Finally, the authors wanted to explore whether risk for ASD-like behaviors in offspring could be induced by exposing the mice without the gut flora (Jax) to the gut flora of the mice who had it (Tac). The idea here was to see if exposure to the gut flora would be sufficient to trigger the IL-17a response in mice who did not previously have it. The authors found that after being exposed to the gut flora, the Jax mice--who originally did NOT have offspring with ASD-like behaviors--began to have offspring who displayed ASD-like behaviors. This is extremely interesting because it suggests that even though these mice do not naturally have gut flora which puts them at risk for the inflammatory response, exposure to the gut flora causes them to have the inflammatory response (and offspring with ASD-like behaviors).
All that said, what does this tell us about autism, and what are the implications for humans?
First, I think it is important to remember that these studies are preliminary, and certainly cannot be taken as direct evidence that these same procedures can be used in humans. However, these studies are a fascinating glimpse into the complex relationship between our immune systems, microbiome (gut), and development of developmental disorders. These types of studies have huge potential for better understanding what circumstances might confer high risk for ASD, and likewise what might work to lessen risk (for example, in these mice it was both specific gut flora AND exposure to a virus during pregnancy to trigger an immune response that caused ASD-like behaviors in offspring--one or the other in isolation did not cause ASD-like behaviors in offspring). ASD is a complex neurodevelopment disorder that almost certainly has multiple causes. Understanding any of these factors will be critical for helping families understand risk and protective factors, and ultimately helping understand what factors contribute to symptoms of ASD.
References
Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. Catherine R. Lammert, Elizabeth L. Frost, Ashley C. Bolte, Matt J. Paysour, Mariah E. Shaw, Calli E. Bellinger, Thaddeus K. Weigel, Eli R. Zunder and John R. Lukens. J Immunol July 2, 2018, ji1701755; DOI: https://doi.org/10.4049/jimmunol.1701755


