Abstract
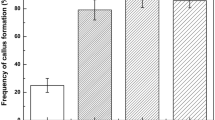
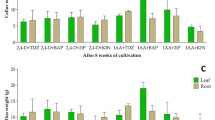
Liquid cultures were successfully generated from cotyledons of two Sonneratia species, S. alba and S. caseolaris in Murashige and Skoog (MS) medium containing 0.1 μmol L−1 2,4-dichlorophenoxyacetic acid (2,4-D). Adventitious roots differentiated from cotyledons of S. alba. Proliferated cells were subcultured and a large volume of suspension cells was subsequently established in 100-mL flasks. All the cytokinins tested inhibited cell proliferation. After three years of culture, the potential to differentiate was tested as indicated by greening of the cells. Greening occurred when suspension cells were transferred to solid MS medium with and without 0.1 μmol L−1 2,4-D. Greening was stimulated by low concentrations of the weak auxins indolebutyric acid (IBA) and naphthaleneacetic acid (NAA) while 2,4-D stimulated late-stage greening. Abscisic acid (ABA) inhibited greening. Gibberellic acid (GA3) at 1.0 μmol L−1 stimulated callus greening and was not inhibitory even when tested at high concentrations. Cytokinins were inhibitory in combination with 0.1 μmol L−1 of either IBA or NAA. The cause of different effects of plant hormones on growth and differentiation was discussed. Small-scale liquid media and 24-well culture plates of solid media methods developed in this paper are suitable for the optimization of hormonal conditions for cell proliferation and differentiation.







Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- BA:
-
Benzyladenine
- CPPU:
-
N-(2-chloro-4-pyridyl)-N′-phenylurea
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- GA3 :
-
Gibberellic acid
- IBA:
-
Indolebutyric acid
- 2-iP:
-
2-iso-Pentenyladenine
- MS:
-
Murashige and Skoog
- NAA:
-
Naphthaleneacetic acid
References
Akatsu M, Hosoi Y, Sasamoto H, Ashihara H (1996) Purine metabolism in cells of a mangrove plant, Sonneratia alba, in tissue culture. J Plant Physiol 149:133–137
Baba S, Onizuka R (1997) Callus induction of five mangrove tree species. In: Bullock A (ed) Mangrove ecosystem studies in Latin America and Africa. UNESCO, France, pp 339–347
Fukumoto T, Nakamura T, Suzuki M, Ogita S, Mimura T, Sasamoto H (2004) Different effects of four salts and pHs on protoplast cultures of a mangrove, Bruguiera sexangula suspension cells, Populus alba leaves and tobacco BY-2 cells. Plant Biotechnol 21:177–182
Kawana Y, Sasamoto H, Mochida Y, Suzuki K (2004) Leaf protoplast isolation from eight mangrove species of three different families; Avicenniaceae, Rhizophoraceae and Sonneratiaceae. Mangrove Sci 3:25–31
Kitamura S, Hachinohe H, Baba S, Hayashi S, Sudana R (1996) Effective seed collections for the genus Sonneratia in Sonneratiaceae (I) fruiting season of Sonneratia alba in Bali of Indonesia and its fruit maturation treatment with bananas (in Japanese). Mangrove Sci 1:51–53
Kura-Hotta M, Mimura M, Tsujimura T, Washitani-Nemoto S, Mimura T (2001) High salt-treatment-induced Na+ extrusion and low salt-treatment-induced Na+ accumulation in suspension-cultured cells of the mangrove plant Bruguiera sexangula. Plant Cell Environ 24:1105–1112
Mimura T, Mimura M, Washitani-Nemoto S, Sakano K, Shimmen T, Siripatanadilok S (1997) Efficient callus initiation from leaf of mangrove plant, Bruguiera sexangula in amino acid medium: effect of NaCl on callus initiation. J Plant Res 110:25–29
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Nakagawa R, Ogita S, Kubo T (2001) Callus culture of mangrove Sonneratia alba (in Japanese). Proc 112th Ann Metg J For Soc 112:271–272
Ogita S, Yeung EC, Sasamoto H (2004) Histological analysis in shoot organogenesis from hypocotyls explants of Kandelia candel (Rhizophoraceae). J Plant Res 117:457–464
Sasamoto H, Hosoi Y, Koshioka M (1995) Endogenous levels of four plant hormones may affect the culture conditions of poplar protoplasts to regenerate plants. In: Terzi M, et al. (eds) Current issues in plant molecular and cellular biology. Kluwer, The Netherlands, pp 481–486
Sasamoto H, Wakita Y, Baba S (1997) Effect of high sorbitol concentration on protoplast isolation from cotyledons of mangroves, Avicennia marina and A. lanata. Plant Biotechnol 14:101–104
Sasamoto H, Ogita S, Mimura T (2000) Protoplast cultures and cell fusion of a mangrove plant, Bruguiera sexangula (in Japanese). Proc 111th Ann Metg J For Soc 111:603
Sasamoto H, Ogita S, Wakita Y, Fukui M (2002) Endogenous levels of abscisic acid and gibberellins in leaf protoplasts competent for plant regeneration in Betula platyphylla and Populus alba. Plant Growth Regul 38:195–201
Satsrinarong M, Okada M, Nishimura K, Ogino K (1992) Study on mangrove tissue culture for micro propagation-Induction of organogenic cultures (in Japanese). Proc 103th Ann Metg J For Soc 103:483
Wakita Y, Sasamoto H, Yokota S, Yoshizawa N (1996) Plant regeneration from cell suspension cultures of Betula platyphylla var. japonica. Plant Tissue Cult Lett 13:49–54
West TP, Preece JE (2006) Use of acephate, benomyl and alginate encapsulation for eliminating culture mites and fungal contamination from in vitro cultures of hardy Hibiscus (Hibiscus moscheutos L.) In Vitro Cell Dev Biol Plant 42:301–304
Yasumoto E, Adachi K, Kato M, Sano H, Sasamoto H, Baba S, Ashihara H (1999) Uptake of inorganic ions and compatible solutes in cultured mangrove cells during salt stress. In Vitro Cell Dev Biol Plant 35:82–85
Acknowledgments
Y.K., R.Y., and H.S. thank Professor E.C. Yeung of the University of Calgary for his critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawana, Y., Yamamoto, R., Mochida, Y. et al. Generation and maintenance of suspension cultures from cotyledons and their organogenic potential of two mangrove species, Sonneratia alba and S. caseolaris . Plant Biotechnol Rep 1, 219–226 (2007). https://doi.org/10.1007/s11816-007-0035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-007-0035-2