1Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia
2Department of Food Irradiation Research, National Centre for Radiation Research and Technology, Cairo, Egypt
Corresponding author email: batoulamjad@gmail.com
Article Publishing History
Received: 05/10/2020
Accepted After Revision: 11/12/2020
In view of the widespread incidence of arsenic poisoning around the world, it was necessary to study this phenomenon and analyze it to find out how to treat it through the application of alternative medicine. Gamma irradiation as a phytosanitary treatment of food and herbal materials is increasingly recognized throughout the world by improving their hygienic quality. The aim of this study was to evaluate the therapeutic effect of raw or irradiated basil on rats exposed to arsenic toxicity. Basil was irradiated by gamma rays at dose 10 KGy. Forty-eight adult Wistar albino rats were divided into six groups as follows group-1: Control group, group-2: received 400 mg/ kg of aqueous extract of basil, group-3: received 400 mg/ kg of aqueous extract of irradiated basil, group- 4: received 10 mg/kg of sodium arsenate solution, group- 5: received 10 mg/kg of sodium arsenate solution and 400mg/ kg of aqueous extract of basil, and group- 6: received 10 mg/kg of sodium arsenate solution and 400mg/ kg of aqueous extract of irradiated basil.
At the end of the experiment (5 weeks), the rats were sacrificed, blood and brain tissue samples were subjected to estimate of the following: CBC, inflammatory markers (CRP, TNF-α, and IL6), immunoglobulin markers (IgA, IgG, IgM), and the levels of oxidative stress and antioxidant in brain tissue (MDA, CAT, SOD, and GSH). The results showed a rise in the antioxidant of basil after the irradiation process. The arsenic caused a significant decreased in levels of HB, RBC, LYM, NEU and PLT and increased levels of WBC and reticulocyte count as compared to the control group. Also, the rats exposed to arsenic showed a significant increase in inflammatory markers, immunoglobulin markers in serum, and oxidative stress accompanied with significant decreased in antioxidant in the brain. In contrast, the administration of basil extract along with arsenic was a helpful factor in alleviation of these side effects. In conclusion, our findings showed that the irradiation process enhanced antioxidants in basil plants which attenuated arsenic toxicity.
Basil, Gamma irradiation, Arsenic, Hematology, Inflammation, Immune System, Brain
Osman N. N, Amjad B. S, Ghazwani A. H, Balamash K. S. Evaluation of the Possible Immunomodulatory and Anti-Inflammatory Effects of Gamma-Irradiated Basil, Ocimum basilicum Against Arsenic Toxicity in Rats. Biosc.Biotech.Res.Comm. 2020;13(4).
Osman N. N, Amjad B. S, Ghazwani A. H, Balamash K. S. Evaluation of the Possible Immunomodulatory and Anti-Inflammatory Effects of Gamma-Irradiated Basil, Ocimum basilicum Against Arsenic Toxicity in Rats. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/34M7zmM
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Arsenic is a toxic substance that occurs naturally and is found in water, rock soil and many foods (Duker et al., 2018).Arsenic toxicity affects millions of people in different parts of the world through the ingestion of arsenic-contaminated drinking water and food (Huq et al., 2006; Flanagan et al., 2012).In recent years, arsenic toxicity has created significant public concern (Duker et al., 2018).It has no taste or smell, which makes it particularly hazardous, so one can be exposed to it without knowing it (ATSDR, 2017). The increasing presence of variable amounts of arsenic in the environment presents major risks, as exposure through inhalation, ingestion and dermal contact can cause various adverse effects on health systems (Vimercati et al., 2017, Chiocchetti et al., 2018 Mochizuki et al., 2019, Tutkun et al., 2019),d (Zhao et al., 2019).
The National Center for CAM (complementary and alternative medicine) was reported as a category of healthcare and medical systems (NCCIH, 2018).A systemic review conducted by Eardley et al. (2012) found that, for many reasons, people are using CAM, including its availability, perceived health, and disease prevention. As one of the main forms of CAM is herbal medicines, which uses parts of a plant or whole plants to avoid and cure diseases (Bent, 2008; Pan et al., 2014).The herbal medicinal products are characterized by the presence of complex chemical compounds responsible for the pharmacological activities that contribute to health benefits(Bent, 2008).Basil plants considered from herbal medicine that has eminence value as a cure for various diseases (Patel et al., 2018).
Basil (Ocimum basilicum L.) is an herbaceous and aromatic plant worldwide cultivated which belongs to the Lamiaceae family (Jakovljević et al., 2016). It has several therapeutic properties including antioxidant, anti-aging, anticancer, antiviral, antimicrobial, anti-genotoxic, and anti-inflammatory(Sakr and Al-Amoudi, 2012; Shirazi et al., 2014). Studies have shown many pharmacological effects for basil in several diseases such as liver fibrosis(Alomar and Al-Attar, 2019), diabetes mellitus (Widjaja and Rusdiana, 2019), asthma (Eftekhar et al., 2019), anemia (Zangeneh et al., 2019), and cerebral injury (Singh, Krishan and Shri, 2018).Based on the pharmacological and therapeutic properties, basil has played an important role in both traditional pharmaceutical products and contemporary pharmacological and clinical science(Shirazi et al., 2014).But there may be concerns about the use of herbs, which is that herbs are susceptible often to contamination during processing or storage by micro-organisms and insect pests. This leads to shortens their shelf life and triggering serious illness in some cases, particularly if the herbs contaminated with Salmonella and Staphylococcus aureus (Chatterjee et al., 2016). Thus, herbs should be subjected to sterilization or microbial treatment before use.
There are various techniques for the decontamination of medicinal plants, including irradiation (Garg and Gupta, 2016).This is a physical process that applies high-energy ionizing radiation to the plants in order to enhance their safety and shelf-life (Byun et al., 1999; SádECká, 2007; Alothman, Bhat and Karim, 2009). In particular, gamma irradiation seemed to be the best way to decontaminate herbs from microbes without triggering quality changes(Lee et al., 2005). As the absorbed energy of radiation can break the bonds of DNA molecules in microorganisms present in the product and inactivates certain enzymes, this greatly reduced its damaging impact on products. Not only microorganisms are destroyed, but even gametes, insects and parasites are prevented from reproducing, resulting in various preservative effects, (Farkas, 2006). Moreover, irradiation serves as a safe technique in food processing supported by many internationally recognized organizations, where joint (Food and Agriculture Organization/ International Atomic Energy Agency/World Health Organization)Expert Committee on the Wholesomeness of Irradiation of Food has ruled that food subject to low irradiation dosage (up to 10 kGy) is safe and not need any testing of toxicology (Wen et al., 2006). The present study was therefore designed to evaluate the possible immunomodulatory and anti-inflammatory effects of raw and irradiated basil (Ocimum basilicum) against arsenic toxicity in rats.
MATERIALS AND METHODS
Chemicals and kits: Sodium arsenate was obtained from British Drug Houses (BDH) chemical company, England, UK. Diethyl ether was obtained from Sigma-Aldrich company, Louis, USA. Kits for assays of immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), tumor necrosis factor-alpha (TNFα), interleukin (IL-6), C- reactive protein (CRP), superoxide dismutase (SOD), glutathione (GSH), catalase (CAT) and malondialdehyde (MDA) were obtained from Abcam Chemical Company, Cambridge, UK. kits for assay Total capacity antioxidant were obtained from Cell Biolabs company,
California, USA. Kits assay protein concentration in tissues (Pierce TM BCA Protein Kit) was obtained from Thermo Fisher Scientific Company, Waltham, US.
Plant material and preparation of extract: The basil (Ocimum basilicum) leaves were purchased from the local traditional market in Jeddah, Saudi Arabia. The water extracts of raw or irradiated dried basil leaves were prepared according to the method described by Ghazwani et al. (2020). The total antioxidant capacity was measured in basil extracts using the Total Capacity Assay kit with CAT# STA-360.
Gamma Irradiation treatment: The samples of dry basil powder were irradiated with 10 KGy of gamma rays after the leaves were transferred into polyethylene bags, using a Cobalt-60 source at a dose rate of 4.75 KGy/h at the National Centre for Radiation Research and Technology (NCRRT), Nasr City, Cairo, Egypt.
Experimental animals and Design: The study was carried out using adult female Wistar rats weighing (150-200g); they were obtained from faculty of pharmacy at King Abdulaziz University. The animals were housed in cages and received normal rat chow and tap water in a constant environment (room temperature 28 ± 2°C, room humidity 60±5%) in a 12 h light and 12 h dark cycle. Rats were kept under supervision for two weeks before the experiments started and during all stages of the whole experiment. Animal’s procedures were performed in accordance with the Ethics Committee of the King Fahad Medical Research Center and in accordance with the recommendations for the proper care and use of laboratory animals. In the experiment, 48 rats were divided into six groups each of 8 rats as follows:
Group 1: normal control rats were given only distilled water. Group 2: rats received 400 mg/ kg of aqueous extract of raw basil (Ezeani et al., 2017). Group 3: rats received 400 mg/ kg of aqueous extract of irradiated basil (Ezeani et al., 2017). Group 4: rats received 10 mg/kg of sodium arsenate solution (Firdaus et al., 2018).Group 5: rats received 10 mg/kg of sodium arsenate solution and 400mg/ kg of aqueous extract of basil. Group 6: rats received 10 mg/kg of sodium arsenate solution and 400mg/ kg of aqueous extract of irradiated basil. All doses were given through an oral gastric tube daily for five weeks. At the end of the experiment, rats fasted overnight for scarification. Blood samples were withdrawn by a heparinized capillary tube from the retro-orbital plexus of each rat under anesthesia with diethyl ether, it is put into two tubes, one is ethylenediamine tetra-acetic acid (EDTA) tube and the other is a serum-separating tube. The EDTA tube immediately turned to lab analysis while serum-separating tube centrifuged at 3000 rpm for 15 min to separate serum and then stored at -40° C until the biochemical analysis is done. Directly after preparing the blood sample, rats sacrificed and the brain was kept in ice for homogenate preparation.
Biochemical analysis: The biochemical analysis for complete blood count (CBC) was done in Kingdoms Labs. The blood serum IgG, IgM and IgA, TNFα, IL-6 and CRP were identified using the kits with CAT# ab189578, ab157735, ab157738, ab46070, ab119548, and ab108827, respectively. For tissue analysis, the brain was homogenized and estimated each of SOD, GSH, CAT and MDA according to the kits with CAT# ab65354, ab138881, ab83464, and ab118970, respectively, while the protein concentration estimated in the brain was used Kits with CAT# 23225.
Statistical analysis: The data of each group were analyzed using MegaStat 9.4 (add-in for Excel). The data were expressed as arithmetic mean and standard deviation of the mean (SD).Differences between groups were analyzed for parametric parameters using one-way variance analysis (ANOVA), the least significant difference equation (LSD). A P value below or equal to 0.05 was considered significant.
RESULTS AND DISCUSSION
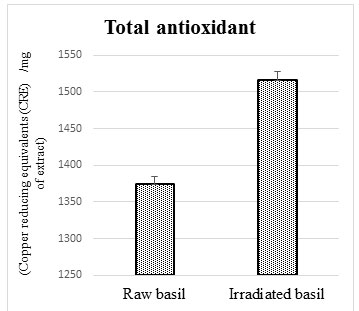
Antioxidant in irradiated plant leaves: The effect of irradiation on antioxidants is showed in figure 1. Where leaves treated with gamma irradiation (10kGy) showed a change, a 10.3% increase in the content of total antioxidants compared to raw leaves not treated with radiation.
Figure 1: Total antioxidants in raw and irradiated basil
Complete blood count (CBC): The effects of aqueous extracts of raw basil or irradiated basil for 5 weeks on the levels of hemoglobin (HB), red blood cells(RBC), hematocrit (HCT), platelets (PLT), White blood cells (WBC), WBC differential and reticulocyte count in rats exposed to arsenic are present in Tables 1 and 2.
No effect of raw or irradiated basil administration in normal animals on all previous parameters was noted. Rats, which were exposed to arsenic showed a significant decrease in levels of HB, RBC, PLT, neutrophils (NEU), and lymphocytes (LYM) compared to the control group. While there was a marked increase in the levels of HCT, WBC, monocytes (MON) and reticulocyte count compared to the control group. Administration of raw and irradiated basil along with arsenic significantly reduced arsenic toxicity effect by the improvement levels of these parameters.
Table 1. Effect of basil extracts on hematological parameters of rats exposed to arsenic
| Test
Group |
HB
(g/dl) |
RBC
(10^ 6 / mL) |
HCT
(% ) |
PLT
(10^ 3 / mL) |
|
| C | 12.661 ±1.258 | 4.450± 0.575 | 29.635± 1.870 | 263.13± 52.58 | |
| B | 12.725 ± 0.902
bbb |
4.570± 0.491
bbb |
29.286± 0.516
bbb |
274.50± 19.17
bbb |
|
| IB | 12.810 ± 0.756
bbb |
4.568 ± 0.487
bbb |
29.518± 0.908
bbb |
277.25± 13.63
bbb |
|
| A | 8.748 ± 0.590
aaa |
3.100 ± 0.524
aaa |
39.058± 0.655
aaa |
143.25± 7.96
aaa |
|
| B + A | 10.918 ± 0.713
aaa bbb |
4.066 ± 0.463
bbb |
30.978± 1.748
bbb |
188.38± 10.89
aaa bb |
|
| IB + A | 11.249 ± 0.290
aabbb |
4.151± 0.164
bbb |
32.330± 1.863
aaa bbb |
177.88± 10.47
aaa bb |
Values are the mean of 8 observation ± SD, Significant different from C value at P < 0.05a, 0.01aa, 0.001aaa,
Significant different from A at value at P < 0.05b, 0.01bb, 0.001bbb
Table 2. Effect of basil extracts on hematological parameters of rats exposed to arsenic
| Test
Group |
WBC
(10^ 3 / mL) |
WBC differential | Reticulocyte
Count (%) |
||
| NEU (%) | LYM (%) | MON (%) | |||
| C | 5.661 ± 0.508 | 62.403 ±1.869 | 28.16 ±2.658 | 4.113±0.631 | 1.413± 0.416 |
| B | 5.255 ± 0.532
bbb |
63.388 ±1.886
bbb |
27.61±4.022
bb |
4.250±0.460
bbb |
1.100± 0.185
bbb |
| IB | 5.134 ± 0.604
bbb |
64.538 ±1.577
bbb |
29.46 ±4.530
bbb |
4.064±0.261
bbb |
1.263± 0.424
bbb |
| A | 9.761± 0.893
aaa |
38.051 ±2.361
aaa |
20.00 ±2.624
aaa |
7.150±0.493
aaa |
2.350± 0.540
aaa |
| B + A | 6.160± 0.825
bbb |
54.175 ±5.389
aaa bbb |
24.95±5.687
b |
5.163±0.460
aaa bbb |
1.011±0.110
bbb |
| IB + A | 5.978± 0.610
bbb |
51.850±5.011
aaa bbb |
25.73±5.035
b |
5.113±0.500
aaa bbb |
1.163±0.518
bbb |
Values are the mean of 8 observation ± SD, Significant different from C value at P < 0.05a, 0.01aa, 0.001aaa,
Significant different from A at value at P < 0.05b, 0.01bb, 0.001bbb
Antibodies: The extracts of raw and irradiated basil had distinct effects on antibodies in rats exposed to arsenic that are showed in Table 3. The treatment with raw or irradiated basil to normal animals not producing an effect on levels of serum IgG, IgM and IgA. The arsenic group showed a significant decrease in IgG, IgM and IgA levels compared to the control group. The treatment with basil extract as raw or irradiated along with arsenic dose was showed a significant increase in those antibodies’ levels, compared with the group of rats given only arsenic.
Table 3. Effect of basil extracts on immunoglobulins of rats exposed to arsenic
| Test
Group |
IgG
(µg/ml) |
IgM
(µg/ml) |
IgA
(µg/ml) |
| C | 517.16 ± 35.137 | 0.628 ± 0.084 | 90.180 ± 6.688 |
| B | 522.490 ± 41.527
bbb |
0.640 ± 0.053
bbb |
93.236 ± 7.697
bbb |
| IB | 514.663 ± 32.665
bbb |
0.655 ± 0.064
bbb |
94.614 ± 5.087
bbb |
| A | 202.500 ± 40.818
aaa |
0.226 ± 0.024
aaa |
45.779 ± 5.816
aaa |
| B + A | 298.911 ± 32.731
aaa bbb |
0.389 ± 0.015
aaa bbb |
72.754 ± 4.942
aaa bbb |
| IB + A | 287.915 ± 35.128
aaa bbb |
0.408 ± 0.021
aaa bbb |
76.745 ± 4.229
aaa bbb |
Values are the mean of 8 observation ± SD, Significant different from C value at P < 0.05a, 0.01aa, 0.001aaa,
Significant different from A at value at P < 0.05b, 0.01bb, 0.001bbb
Inflammatory markers:The level of markers of inflammation in rats exposed to arsenic which received raw or irradiated basil as a treatment are showed in Table 4.
In normal animals, the administration of raw or irradiated basil has not had an effect on the levels of serum TNF, IL-6 and CPR. The rats exposed to arsenic showed a significant increase in levels of TNF, IL-6, and CPR as compared to the control group. Giving raw or irradiated basil extract significantly reduced inflammatory markers induced by arsenic.
Table 4. Effect of basil extracts on the inflammation markers of rats exposed to arsenic
| Test
Group |
TNFα
(pg/ml) |
IL-6
(pg/ml) |
CRP
(ng/ml) |
| C | 17.829 ± 1.221 | 21.048 ± 2.134 | 0.134 ± 0.039 |
| B | 16.144 ± 1.428
bbb |
20.753 ± 1.962
bbb |
0.134± 0.034
bbb |
| IB | 16.123 ± 1.216
bbb |
20.456 ± 2.940
bbb |
0.140± 0.018
bbb |
| A | 40.043 ± 2.453
aaa |
35.334 ± 3.973
aaa |
0.990± 0.076
aaa |
| B + A | 27.656 ± 2.173
aaabbb |
27.413 ± 3.842
aaabbb |
0.660± 0.093
aaabbb |
| IB + A | 28.935 ± 2.634
aaabbb |
26.876 ± 1.563
aaabbb |
0.490± 0.033
aaabbb |
Values are the mean of 8 observation ± SD, Significant different from C value at P < 0.05a, 0.01aa, 0.001aaa,
Significant different from A at value at P < 0.05b, 0.01bb, 0.001bbb
Antioxidants and oxidative damage: The results of determining the content of SOD, GSH, CAT and MDA in rats given arsenic along with raw basil or irradiated basil were shown in Table 5.The normal rats treated with irradiated basil showed an increase in the level of SOD than rats treated with raw basil. The level of GSH was showed an increase in normal rats of both of raw basil group and irradiated basil group as compared to the control group. Arsenic-exposed rats showed a significant increase in MDA levels accompanied by a significant decrease in SOD, GSH and CAT compared to a control group. The management of raw or irradiated basil along with arsenic alleviated the effects of arsenic and resulted in a significantly decreased MDA with significantly increased SOD, GSH, CAT.
Table 5. Effect of basil extracts on the antioxidants and oxidative damage in the brain of rats exposed to arsenic
| Test
Group |
SOD
(Inhibition rate %/ mg of protein) |
GSH
(µM/gram tissue) |
CAT
(µM/mg of protein) |
MDA (µM/mg of
protein) |
| C | 105.406 ±7.342 | 90.743 ± 7.044 | 0.020 ± 0.003 | 2.496± 0.451 |
| B | 107.548 ±9.384
bbb |
96.709 ± 3.420
abbb |
0.019 ± 0.004
bbb |
2.400± 0.422
bbb |
| IB | 114.655 ±8.165
abbb |
95.425 ± 4.426
abbb |
0.021 ± 0.004
bbb |
2.441± 0.433
bbb |
| A | 77.977 ±10.370
aaa |
60.248 ± 2.657
aaa |
0.009 ± 0.000
aaa |
6.005± 0.126
aaa |
| B + A | 82.049 ±8.953
aaabbb |
84.259 ± 4.487
aabbb |
0.012 ± 0.001
aaa |
3.479± 0.610
aaabbb |
| IB + A | 89.249 ±9.381
aaabbb |
82.533 ± 3.153
aabbb |
0.014 ± 0.003
aabb |
3.165± 0.484
aabbb |
Values are the mean of 8 observation ± SD, Significant different from C value at P < 0.05a, 0.01aa, 0.001aaa,
Significant different from A at value at P < 0.05b, 0.01bb, 0.001bbb
The irradiation treatment can increase the content of some phytochemicals and the plant’s antioxidant activity, thereby increased biological value (Zevallos-Concha et al., 2016; Pereira et al., 2018).The results indicated that the extract of irradiated basil showed a highly significant increase in total antioxidants as a comparison to raw basil. Similar observations were reported in previous studies on basil and some other plants (Khawory et al., 2020; Osman et al., 2020; Rady et al., 2020).
Basil contains phenolic compounds and flavonoids (Bahcesular et al., 2020), that are considered as natural antioxidants. These biomolecules exhibit their activity through various mechanisms, including inhibiting enzymes that inducing free radical produce, increasing endogenous antioxidants, removing free radicals, and inducing the expression of the numerous genes responsible for enzyme synthesis that inhibit oxidative stress (Primiano, Sutter and Kensler, 1997). Ghazwani, Osman and Balamash (2020) have recently reported that the Fourier-transform infrared (FTIR) analysis indicated to increase the content of phenolic acids and flavonoids in basil leaves after treated with 10 kGy of gamma-ray. Moreover, Maraei, Khaled and Elsawy (2017)reported that the gamma irradiation-induced the biosynthesis of certain phenolic compounds. Also, it seems that gamma irradiation with 10 kGy might stimulate some chemical reactions in basil, which perhaps increase in phenolic content by the breakdown of covalence bonds among phenolic components and, free phenolic components with low molecular weight are increasing (Jamshidi, Barzegar and Sahari, 2014).
A complete blood count test is a blood test used to assess general health and detect a range of disorders in hematological parameters. A complete blood count test measures many blood components and features, including red blood cells that carry oxygen, white blood cells that fight infection, hemoglobin that oxygen-carrying protein in red blood cells, hematocrit that indicate to the ratio of red blood cells to the liquid or plasma component of the blood and platelets that help blood clot, and that any change whether an abnormal rise or decrease in the census, indicate the incidence of diseases or disorders requiring medical procedures(Clinic, 2018).
In our study, it has been observed that the levels of HB, RBC, PLT, NEU, and LYM are decreased significantly with a marked increase in the HCT, WBC, MON and reticulocyte count in rats exposed to arsenic compared to the control group. The results are consistent with some studies (Kajiguchi et al., 2005; Bhattacharya and Haldar, 2012; Sumedha and Miltonprabu, 2013; Lemaire et al., 2015; Ghosh et al., 2017; Su et al., 2018).This effect of Arsenic exposure on the hematopoietic system may be attributed to the mechanisms of arsenic toxicity which may induce hemolysis and erythrophagocytosis through increased oxidation of sulfhydryl groups in hemoglobin and decreased oxygen intake by cells as a result of decreased intracellular glutathione, which decreases the lifespan of erythrocytes(Abdul et al., 2015). Moreover, arsenic exposure can also cause a range of changes, such as increasing ceramide formation, membrane disintegration, cytosolic calcium levels, besides decreasing in adenosine triphosphate (ATP) levels, cell membrane integrity affecting erythrocyte lifespan (Abdul et al., 2015).
Regarded the change in platelet count, this confirmed that arsenic inhibited platelet differentiation within the hematopoietic system of bone marrow, leading to reduced platelet production(Wu et al., 2014).The white blood cell level was decreased in arsenic feed groups. It might be due to the impact of arsenic which induced apoptotic effect on plasma cells as noted by Rousselot et al. (2004). WBC may generally be divided into five classes, based on their function, morphology and origin: LYM, MON and NEU(Villa et al., 2003). The changes in the LYM and NEU populations present in this study may be due to arsenic caused immune inhibition in rats (Taheri et al., 2016).
Our findings demonstrated that extracts of basil caused improved the disorders that occur in CBC. This is in agreement with the results of previous researches (Ofem et al., 2012; Zangeneh et al., 2019).This effect may due to basil which contains a proportion of iron (Nworgu, Yekini and Oduola, 2013), that contributes to improving the level of HB in the blood and has the ability to stimulate production and increase of RBC to treat deficiency caused by arsenic. Furthermore, in normal, the lack of oxygen in the local tissue appears to lead to the production of glycoprotein known as erythropoietin, which induces increased erythrocyte output(Bowman and Rand, 1980).Basil leaves extract is very likely to contain erythropoietin-like agents that are responsible for increased erythrocyte production (Ofem, Ani and Eno, 2012). Saha et al. (2012)reported that secondary metabolites of basil, consisting of important elements include essential oil geraniol, a monoterpene and citral, play a role as the modulator in hematological abnormalities (Ofem et al., 2012). Moreover, in results about the increased lymphocyte count after basil administration, it has been reported that Ocimum basilicum modulates the cell-mediated as well as a humoral immune response that could be due to the presence of flavonoids and terpenoids (Mediratta et al., 2002).
Antibodies also are known as immunoglobulins, are substances made by the body’s immune system in response to foreign substances. Antibodies bind to these foreign substances and they can be killed by the immune system. IgG, IgM and IgA from the major types of antibodies. If reduced levels of antibodies produced by the immune system, it leads to more likely to develop repeated infections(Staff, 2019).
The results of our study showed that in the arsenic group of rats (10 mg/kg BW) a highly significant reduction was observed in IgG, IgA and IgM levels compared to the control group. The results are consistent with some studies (Institoris et al., 2001; Sankar et al., 2013). The effect on immunoglobulin levels associated with arsenic exposure can attribute to the arsenic disrupts glucocorticoid regulation, responsible for immune function, (Kaltreider et al., 2001). Furthermore, the apoptosis caused by arsenic may result in decreased immune responses(Harrison and McCoy, 2001).The administration of water extract from raw or irradiated basil extract demonstrated a protective effect against arsenic toxicity in rats, by increasing antibodies that decreased as a result of arsenic poisoning. These results are in line with the findings of Mohammed, Kadhim and Taher (2017) and Jahejo et al. (2019). Jeba, Vaidyanathan and Rameshkumar (2011)showed that aqueous extract of basil stimulated the antibody production in rats. The flavonoids present in the basil leaves are mainly responsible for the immunomodulatory effect (Ravindran, 2017).
Inflammation is a biological response of the immune system which can be induced by damaged cells, toxic compounds or pathogens (Medzhitov, 2010). It is part of the body’s defense mechanism. one of the major aims of inflammation is to bring immune cells to the area of concern as well as to inactivate or destroy any injurious stimuli and to also begin the repair(Ferrero-Miliani et al., 2007; Medzhitov, 2010).The inflammation response is caused by specific immune factors released from the damaged cells. Where, the damaged cells release cytokines, including interleukins, such as IL-6, IL-8, and tumor necrosis factor-α, that are responsible for communication between white blood cells. Interleukins also stimulate the production and release of CRP from the liver; an important component of the innate immune system (Sinclair et al., 2012).
Usually, molecular and cellular activities and interactions efficiently alleviate inevitable infection or damage, during acute inflammatory responses. This effect helps restore homeostasis in the tissue and overcome the acute inflammation. Uncontrolled acute inflammation can become chronic, however, and can lead to a number of chronic inflammatory diseases (Zhou et al., 2016). The elevated levels of inflammatory markers are expected to be associated with toxic metals exposure.Results of this study showed that serum IL-6, TNF-α and CRP level in rats exposed to arsenic was highly significantly elevated. Our findings agree with the results of the previous study on the association between arsenic and ability to cause chronic inflammation by demonstrating the increased pro-inflammatory mediators like TNF-α, IL-6 and CRP in the arsenic exposed group in comparison to the control group(Prasad and Sinha, 2017).Inflammation considered to be one of the main arsenic toxicity mechanisms that can be correlated with increasing cellular damages ,oxidative stress and lipid peroxidation (Bhadauria and Flora, 2007).
In this work also, it was demonstrated that oral treatment with basil extracts diminished inflammations in rats exposed to arsenic. These results in agreement with Aye et al. (2019) and Takeuchi et al. (2020) who found that basil has anti-inflammatory effects. Rodrigues et al. (2016) reported that the basil essential oil was effective in reducing inflammations (acute or chronic) by induced inhibiting of the inflammatory mediator receptors and the migration of cells to stimulus locations. This may be attributed to the contains of basil of rosmarinic acid (Kwon et al., 2019), where this compound has been related to anti-inflammatory activities (Luo et al., 2020).
Antioxidants are considered the enzymes of the body’s protection, are able to stabilize free radicals before attacking components of the cell. They work to diminishing free radicals through reducing their energy or donate electrons to them, thus making it stable(Krishnamurthy and Wadhwani, 2012). While, “oxidative stress, defined as a disturbance in the balance between the production of reactive oxygen species (free radicals) and antioxidant defenses”(Betteridge, 2000).In the present study, observed a very highly significant decrease in GSH, SOD and CAT accompanied by increased MDA of the rat’s brain in the arsenic group as compared to the normal control group. This result is similar to the study of Sun et al. (2018)that reported that arsenic caused significantly decreased GSH, SOD and CAT with increased MDA content in the brain tissues of chickens. This may be attributed to the toxic effect of arsenic that may induce oxidative stress by interacting with antioxidants, resulting in the accumulation of free radicals in cells (Bonetto, Villaamil Lepori and Puntarulo, 2014).
In contrast, basil giving a positive effect on the brain by improving the levels of antioxidant antioxidants, and fat oxidation (MDA).These results were in line with recent studies showed that the water extract of gamma-irradiated basil contributes to improving the oxidative stress induced by arsenic exposure in rats (Ghazwani, Osman and Balamash, 2020; Osman, Ghazwani and Balamash, 2020).Alsoagree with the results of Khodabakhshi et al. (2017), who proved that the increased level of MDA in the mice brain tissue following seizures was prevented by basil extract. Moreover, Khaki (2016) demonstrated that the basil extract protects brain cells from the harmful effects by regulating the antioxidant enzymes in the serum. This saves the neurons from irreversible cell injury. The antioxidant effect is due primarily to phenolic elements, such as, phenolic acids and flavonoids, which have redox properties and ability to neutralize free radicals (Shahidi et al., 1992).
CONCLUSION
In this study, we demonstrated that gamma radiations have the ability to increases antioxidants contents in basil leaves. Moreover, the water extracts obtained from Ocimum basilicum can be successful in diminished arsenic toxic effect through the improvement of the homeostasis of CBC and immunoglobins level, reduced inflammatory markers and, enhancement ability of antioxidants to be overcome oxidative stress in the brain.
Disclosure statement: No potential conflict of interest was reported by the authors.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Ethics Committee of the King Fahad Medical Research Center and in accordance with the recommendations for the proper care and use of laboratory animals.
REFERENCES
Abdul, K. S. M., Jayasinghe, S. S., Chandana, E. P., Jayasumana, C. and De Silva, P. M. C. (2015), Arsenic and human health effects: A review, Environmental toxicology and pharmacology, vol.40: 828-846.
Alomar, M. Y. and Al-Attar, A. M. (2019), Effect of Basil Leaves Extract on Liver Fibrosis Induced by Thioacetamide in Male Rats, International Journal of Pharmacology, vol.15: 478-485.
Alothman, M., Bhat, R. and Karim, A. (2009), Effects of radiation processing on phytochemicals and antioxidants in plant produce, Trends in Food Science & Technology, vol.20: 201-212.
ATSDR (2017), ATSDR’s Substance Priority List, Atlanta, Georgia: Agency for Toxic Substances and Disease Registry.
Aye, A., Jeon, Y.-D., Lee, J. H., Bang, K. S. and Jin, J. S. (2019), Anti-inflammatory activity of ethanol extract of leaf and leaf callus of basil (Ocimum basilicum L.) on RAW 264.7 macrophage cells, Oriental Pharmacy and Experimental Medicine, vol.19: 217-226.
Bahcesular, B., Yildirim, E. D., Karaçocuk, M., Kulak, M. and Karaman, S. (2020), Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress, Industrial Crops and Products, vol.146: 112165.
Bent, S. (2008), Herbal medicine in the United States: review of efficacy, safety, and regulation, Journal of general internal medicine, vol.23: 854-859.
Betteridge, D. J. (2000), What is oxidative stress?, Metabolism-Clinical and Experimental, vol.49: 3-8.
Bhadauria, S. and Flora, S. (2007), Response of arsenic-induced oxidative stress, DNA damage, and metal imbalance to combined administration of DMSA and monoisoamyl-DMSA during chronic arsenic poisoning in rats, Cell biology and toxicology, vol.23: 91-104.
Bhattacharya, S. and Haldar, P. K. (2012), Ameliorative effect Trichosanthes dioica root against experimentally induced arsenic toxicity in male albino rats, Environmental toxicology and pharmacology, vol.33: 394-402.
Bonetto, J. G., Villaamil Lepori, E. C. and Puntarulo, S. Á. (2014), Update on the oxidative stress associated with arsenic exposure, Current Topics in Toxicology, vol.10: 37-47.
Bowman, W. C. and Rand, M. J. (1980), Textbook of pharmacology: Blackwell Scientific Publications.
Byun, M.-W., Yook, H.-S., Kim, K.-S. and Chung, C.-K. (1999), Effects of gamma irradiation on physiological effectiveness of Korean medicinal herbs, Radiation Physics and Chemistry, vol.54: 291-300.
Chatterjee, S., Kumar, V., Khole, S., Sanyal, B., Murali, T. and Variyar, P. S. (2016), Radiation processing: An effective quality control tool for hygienization and extending shelf life of a herbal formulation, Amritamehari churnam, Journal of Radiation Research and Applied Sciences, vol.9: 86-95.
Chiocchetti, G. M., Vélez, D. and Devesa, V. (2018), Effect of subchronic exposure to inorganic arsenic on the structure and function of the intestinal epithelium, Toxicology letters, vol.286: 80-88.
Clinic, M. (2018), Complete blood count (CBC), From: https://www.mayoclinic.org/tests-procedures/complete-blood-count/about/pac-20384919. Access date; Apr. 14, 2020, 2020.
Duker, A., Ndur, S., Osei EMJnr, A. I. and Apedo, G. (2018), Drinking Arsenic Water May Contribute to Mycobac-terium ulcerans, Archives of Epidemiology, vol.2018: 7.
Eardley, S., Bishop, F. L., Prescott, P., Cardini, F., Brinkhaus, B., Santos-Rey, K., Vas, J., von Ammon, K., Hegyi, G. and Dragan, S. (2012), A systematic literature review of complementary and alternative medicine prevalence in EU, Complementary Medicine Research, vol.19: 18-28.
Eftekhar, N., Moghimi, A., Roshan, N. M., Saadat, S. and Boskabady, M. H. (2019), Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma, BMC Complementary and Alternative Medicine, vol.19: 349.
Ezeani, C., Ezenyi, I., Okoye, T. and Okoli, C. (2017), Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes, Journal of Intercultural Ethnopharmacology, vol.6: 22-28.
Farkas, J. (2006), Irradiation for better foods, Trends in food science & technology, vol.17: 148-152.
Ferrero-Miliani, L., Nielsen, O., Andersen, P. and Girardin, S. (2007), Chronic inflammation: importance of NOD2 and NALP3 in interleukin‐1β generation, Clinical & Experimental Immunology, vol.147: 227-235.
Firdaus, F., Zafeer, M. F., Waseem, M., Ullah, R., Ahmad, M. and Afzal, M. (2018), Thymoquinone alleviates arsenic induced hippocampal toxicity and mitochondrial dysfunction by modulating mPTP in Wistar rats, Biomedicine & Pharmacotherapy, vol.102: 1152-1160.
Flanagan, S. V., Johnston, R. B. and Zheng, Y. (2012), Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation, Bulletin of the World Health Organization, vol.90: 839-846.
Garg, N. and Gupta, P. C. (2016), Irradiation: a Technique for microbial Decontamination of medicinal Plants: Springer.
Ghazwani, A. H., Osman, N. N. and Balamash, K. S. (2020), Role of Gamma-irradiated Basil (Ocimum basilicum) in the Alleviation of Heart Toxicity Induced by Arsenic in Rats, International Journal of Pharmaceutical and Phytopharmacological “in press”.
Ghosh, S., Mishra, R., Biswas, S., Bhadra, R. K. and Mukhopadhyay, P. K. (2017), α-Lipoic acid mitigates arsenic-induced hematological abnormalities in adult male rats, Iranian journal of medical sciences, vol.42: 242–250.
Harrison, M. T. and McCoy, K. L. (2001), Immunosuppression by arsenic: a comparison of cathepsin L inhibition and apoptosis, International immunopharmacology, vol.1: 647-656.
Huq, S. I., Joardar, J., Parvin, S., Correll, R. and Naidu, R. (2006), Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation, Journal of health, population, and nutrition, vol.24: 305-316.
Institoris, L., Siroki, O., Űndeger, Ű., Basaran, N. and Desi, I. (2001), Immunotoxicological investigation of subacute combined exposure by permethrin and the heavy metals arsenic (III) and mercury (II) in rats, International immunopharmacology, vol.1: 925-933.
Jahejo, A. R., Rajput, N., Wen-xia, T., Naeem, M., Kalhoro, D. H., Kaka, A., Niu, S. and Jia, F.-j. (2019), Immunomodulatory and Growth Promoting Effects of Basil (Ocimum basilicum) and Ascorbic Acid in Heat Stressed Broiler Chickens, Pakistan Journal of Zoology, vol.51: 801-807.
Jakovljević, Z., Topuzović, D., Bojović, B. and Stanković, M. S. Characteristics of germination and biomass production of Ocimum basilicum L. cultured in vitro. The 21th Conference about Biotechnology with international participation, Čačak, Serbia, Conference Proceeding, 2016. 663-666.
Jamshidi, M., Barzegar, M. and Sahari, M. (2014), Effect of gamma and microwave irradiation on antioxidant and antimicrobial activities of Cinnamomum zeylanicum and Echinacea purpurea, International Food Research Journal, vol.21.
Jeba, C., Vaidyanathan, R. and Rameshkumar, G. (2011), Immunomodulatory activity of aqueous extract of Ocimum sanctum in rat, International Journal on Pharmaceutical and Biomedical Research, vol.2: 33-38.
Kajiguchi, T., Yamamoto, K., Sawa, M., Emi, N. and Naoe, T. (2005), Increased erythropoietin level and reticulocyte count during arsenic trioxide therapy, Leukemia, vol.19: 1-3.
Kaltreider, R. C., Davis, A. M., Lariviere, J. P. and Hamilton, J. W. (2001), Arsenic alters the function of the glucocorticoid receptor as a transcription factor, Environmental health perspectives, vol.109: 245-251.
Khaki, A. (2016), Protective Effect of ocimum basilicum on brain cells exposed to oxidative damage by electromagnetic field in rat: ultrastructural study by transmission electron microscopy, Crescent Journal of Medical and Biological Sciences, vol.3: 1-7.
Khawory, M. H., Sain, A. A., Rosli, M. A. A., Ishak, M. S., Noordin, M. I. and Wahab, H. A. (2020), Effects of gamma radiation treatment on three different medicinal plants: Microbial limit test, total phenolic content, in vitro cytotoxicity effect and antioxidant assay, Applied Radiation and Isotopes, vol.157: 109013.
Khodabakhshi, T., Beheshti, F., Hosseini, M., Mousavi, S. M., Rakhshandeh, H., Sadeghnia, H. R. and Aghaei, A. (2017), Effect of Ocimum basilicum hydro-alcoholic extract on oxidative damage of brain tissue following seizures induced by pentylenetetrazole in mice, Physiology and Pharmacology, vol.21: 295-303.
Krishnamurthy, P. and Wadhwani, A. (2012), Antioxidant enzymes and human health, Antioxidant enzyme, 1-17.
Kwon, D. Y., Li, X., Kim, J. K. and Park, S. U. (2019), Molecular cloning and characterization of rosmarinic acid biosynthetic genes and rosmarinic acid accumulation in Ocimum basilicum L, Saudi journal of biological sciences, vol.26: 469-472.
Lee, J. H., Lee, K. T. and Kim, M. R. (2005), Effect of gamma‐irradiated red pepper powder on the chemical and volatile characteristics of kakdugi, a Korean traditional fermented radish kimchi, Journal of food science, vol.70: c441-c447.
Lemaire, M., Silva, L. F. N., Lemarie, C. A., Bolt, A. M., Molina, M. F., Krohn, R. M., Smits, J. E., Lehoux, S. and Mann, K. K. (2015), Arsenic exposure increases monocyte adhesion to the vascular endothelium, a pro-atherogenic mechanism, PloS one, vol.10.
Luo, C., Zou, L., Sun, H., Peng, J., Gao, C., Bao, L., Ji, R., Jin, Y. and Sun, S. (2020), A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases, Frontiers in Pharmacology, vol.11: 153.
Maraei, R. W., Khaled, M. and Elsawy, K. M. (2017), Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation, Journal of Radiation Research and Applied Sciences, vol.10: 80-87.
Mediratta, P., Sharma, K. and Singh, S. (2002), Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action, Journal of Ethnopharmacology, vol.80: 15-20.
Medzhitov, R. (2010), Inflammation 2010: new adventures of an old flame, Cell, vol.140: 771-776.
Mochizuki, H., Phyu, K. P., Aung, M. N., Zin, P. W., Yano, Y., Myint, M. Z., Thit, W. M., Yamamoto, Y., Hishikawa, Y. and Thant, K. Z. (2019), Peripheral neuropathy induced by drinking water contaminated with low-dose arsenic in Myanmar, Environmental Health and Preventive Medicine, vol.24: 1-10.
Mohammed, Z. I., Kadhim, K. S. and Taher, M. G. (2017), Effects of feeding different levels of Ocimum basilicum seeds on per-formance and immune traits of broiler, Journal of Kerbala for Agricultural Sciences, vol.4: 249-258.
NCCIH. (2018), Complementary, Alternative, or Integrative Health: What’s In a Name?, From: https://nccih.nih.gov/health/integrative-health. Access date; 28 December, 2019.
Nworgu, F., Yekini, B. and Oduola, O. (2013), Effects of basil leaf (Ocimum gratissimum) supplement on some blood parameters of growing pullets, International Journal of Agriculture, vol.3: 480-488.
Ofem, O., Ani, E. and Eno, A. (2012), Effect of aqueous leaves extract of Ocimum gratissimum on hematological parameters in rats, International Journal of Applied and Basic Medical Research, vol.2: 38-42.
Osman, N. N., Ghazwani, A. H. and Balamash, K. S. (2020), Evaluation of the effect of gamma-irradiated Basil (Ocimum basilicum L.) on Liver Toxicity induced by Arsenic in Rats Journal of Radiation Research and Applied Sciences (in press).
Pan, S.-Y., Litscher, G., Gao, S.-H., Zhou, S.-F., Yu, Z.-L., Chen, H.-Q., Zhang, S.-F., Tang, M.-K., Sun, J.-N. and Ko, K.-M. (2014), Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources, Evidence-Based Complementary and Alternative Medicine, vol.2014: 20.
Patel, M., Gadhvi, H., Patel, S., Mankad, A., Pandya, H. and Rawal, R. (2018), Holy basil: Holy herb to multimodal medicine for human health, The Pharma Innovation Journal, vol.7: 418-423.
Pereira, E., Pimenta, A. I., Barros, L., Calhelha, R. C., Antonio, A. L., Verde, S. C. and Ferreira, I. C. (2018), Effects of gamma radiation on the bioactivity of medicinal and aromatic plants: Mentha piperita L., Thymus vulgaris L. and Aloysia citrodora Paláu as case studies, Food & function, vol.9: 5150-5161.
Prasad, P. and Sinha, D. (2017), Low-level arsenic causes chronic inflammation and suppresses expression of phagocytic receptors, Environmental Science and Pollution Research, vol.24: 11708-11721.
Primiano, T., Sutter, T. R. and Kensler, T. W. (1997), Redox regulation of genes that protect against carcinogens, Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, vol.118: 487-497.
Rady, A. H., Toliba, A. O., Badr, H. M. and Ali, A. K. (2020), Impact of gamma radiation on antioxidant activity in faba bean (Vicia faba L.) and the potential of meatballs formulation with inclusion of the powder of irradiated beans, Journal of Food Science and Technology, 1-10.
Ravindran, P. (2017), The encyclopedia of herbs and spices: CABI.
Rodrigues, L. B., Martins, A. O. B. P. B., Cesário, F. R. A. S., e Castro, F. F., de Albuquerque, T. R., Fernandes, M. N. M., da Silva, B. A. F., Júnior, L. J. Q., da Costa, J. G. M. and Coutinho, H. D. M. (2016), Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: in vivo mouse models, Chemico-biological interactions, vol.257: 14-25.
Rousselot, P., Larghero, J., Arnulf, B., Poupon, J., Royer, B., Tibi, A., Madelaine-Chambrin, I., Cimerman, P., Chevret, S. and Hermine, O. (2004), A clinical and pharmacological study of arsenic trioxide in advanced multiple myeloma patients, Leukemia, vol.18: 1518-1521.
SádECká, J. (2007), Irradiation of spices–a review, Czech Journal of Food Sciences vol.25: 231-242.
Saha, S., Mukhopadhyay, M., Ghosh, P. and Nath, D. (2012), Effect of methanolic leaf extract of Ocimum basilicum L. on benzene-induced hematotoxicity in mice, Evidence-Based Complementary and Alternative Medicine, vol.2012.
Sakr, S. A. and Al-Amoudi, W. M. (2012), Effect of leave extract of Ocimum basilicum on deltamethrin induced nephrotoxicity and oxidative stress in albino rats, Journal of Applied Pharmaceutical Science, vol.2: 22.
Sankar, P., Telang, A. G., Suresh, S., Kesavan, M., Kannan, K., Kalaivanan, R. and Sarkar, S. N. (2013), Immunomodulatory effects of nanocurcumin in arsenic-exposed rats, International immunopharmacology, vol.17: 65-70.
Shahidi, F., Janitha, P. and Wanasundara, P. (1992), Phenolic antioxidants, Critical reviews in food science & nutrition, vol.32: 67-103.
Shirazi, M. T., Gholami, H., Kavoosi, G., Rowshan, V. and Tafsiry, A. (2014), Chemical composition, antioxidant, antimicrobial and cytotoxic activities of T agetes minuta and Ocimum basilicum essential oils, Food science & nutrition, vol.2: 146-155.
Sinclair, R. R., Wang, M. and Tetrick, L. E. (2012), Research methods in occupational health psychology: Measurement, design, and data analysis, New York: Routledge.
Singh, V., Krishan, P. and Shri, R. (2018), Improvement of memory and neurological deficit with Ocimum basilicum L. extract after ischemia reperfusion induced cerebral injury in mice, Metabolic brain disease, vol.33: 1111-1120.
Staff, H. (2019), Immunoglobulins, From: https://www.uofmhealth.org/health-library/hw41342. Access date; March 19, 2020.
Su, M., Sun, C., Wang, H., Yuan, C., Guo, R., Liang, Y., Liu, C. and Wang, Q. (2018), Hematotoxicity of intratracheally instilled arsenic trioxide in rats, Infection International, vol.6: 32-40.
Sumedha, N. and Miltonprabu, S. (2013), Arsenic induced oxidative hematotoxicity in rats and its protection by diallyl trisulfide, International Journal of Biological & Pharmaceutical Research, vol.4: 507-515.
Sun, X., Li, J., Zhao, H., Wang, Y., Liu, J., Shao, Y., Xue, Y. and Xing, M. (2018), Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus, Journal of inorganic biochemistry, vol.178: 54-62.
Taheri, M., Mehrzad, J., Afshari, R., Saleh-Moghaddam, M. and Mahmudy Gharaie, M. H. (2016), Inorganic arsenic can be potent granulotoxin in mammalian neutrophils in vitro, Journal of immunotoxicology, vol.13: 686-693.
Takeuchi, H., Takahashi-Muto, C., Nagase, M., Kassai, M., Tanaka-Yachi, R. and Kiyose, C. (2020), Anti-inflammatory Effects of Extracts of Sweet Basil (Ocimum basilicum L.) on a Co-culture of 3T3-L1 Adipocytes and RAW264. 7 Macrophages, Journal of Oleo Science, ess19321.
Tutkun, L., Gunduzoz, M., Turksoy, V. A., Deniz, S., Oztan, O., Cetintepe, S. P., Iritas, S. B. and Yilmaz, F. M. (2019), Arsenic-induced inflammation in workers, Molecular Biology Reports, vol.46: 2371-2378.
Villa, G., Córdova, A., Ávila, C., Almar, M., Marroyo, J., García, J. and del Villar, V. (2003), Modificaciones de los leucocitos en ciclistas profesionales a lo largo de la competición, Revista clinica espanola, vol.203: 412-416.
Vimercati, L., Gatti, M. F., Gagliardi, T., Cuccaro, F., De Maria, L., Caputi, A., Quarato, M. and Baldassarre, A. (2017), Environmental exposure to arsenic and chromium in an industrial area, Environmental Science and Pollution Research, vol.24: 11528-11535.
Wen, H.-W., Chung, H.-P., Chou, F.-I., Lin, I.-h. and Hsieh, P.-C. (2006), Effect of gamma irradiation on microbial decontamination, and chemical and sensory characteristic of lycium fruit, Radiation Physics and Chemistry, vol.75: 596-603.
Widjaja, S. S. and Rusdiana, M. S. (2019), Glucose Lowering Effect of Basil Leaves in Diabetic Rats, Open Access Macedonian Journal of Medical Sciences, vol.7: 1415-1417.
Wu, Y., Dai, J., Zhang, W., Yan, R., Zhang, Y., Ruan, C. and Dai, K. (2014), Arsenic trioxide induces apoptosis in human platelets via C-Jun NH2-terminal kinase activation, PloS one, vol.9.
Zangeneh, M. M., Zangeneh, A., Salmani, S., Jamshidpour, R. and Kosari, F. (2019), Protection of phenylhydrazine-induced hematotoxicity by aqueous extract of Ocimum basilicum in Wistar male rats, Comparative Clinical Pathology, vol.28: 331-338.
Zevallos-Concha, A., Nuñez, D., Gasco, M., Vasquez, C., Quispe, M. and Gonzales, G. (2016), Effect of gamma irradiation on phenol content, antioxidant activity and biological activity of black maca and red maca extracts (Lepidium meyenii walp), Toxicology mechanisms and methods, vol.26: 67-73.
Zhao, Y., Su, X., Gao, Y., Yin, H., Wang, L., Qiao, R. and Wang, S. (2019), Exposure of low concentration arsenic initiated inflammation and autophagy in rat lungs, Journal of biochemical and molecular toxicology, 1-7.
Zhou, Y., Hong, Y. and Huang, H. (2016), Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway, Kidney and Blood Pressure Research, vol.41: 901-910.