processing....
With the number of Americans with cataracts estimated to increase to 30.1 million, or nearly 10% of the US population, by 2020,[1] the demand for a safe and effective surgery will only continue to grow over the next decade. One of the keys to a successful operation is the prevention of complications, which, although rare and ranging from mild and transient to vision-threatening, still occur in approximately 5% of patients.[2] Infection, in the form of endophthalmitis, and inflammation, which can lead to cystoid macular edema (CME), are the 2 major complications of cataract surgery, requiring preventive measures. Most physicians use a prophylactic perioperative regimen consisting of topical fluoroquinolones, corticosteroids, and nonsteroidal anti-inflammatory drugs (NSAIDs) to reduce the risk for infection and inflammation. This article reviews some of the most commonly employed pharmacologic strategies.
Despite advances in surgical techniques, the incidence of endophthalmitis after cataract surgery has paradoxically increased at last report.[3] Clinical endophthalmitis is most often caused by microorganisms introduced into the eye from the patient's conjunctival flora,[4] but they can also originate from external sources, such as contaminated instruments, disposable supplies, prepared solutions, the surgical field, or intraocular lenses.[5,6] Anti-infectives are widely used for surgical prophylaxis in the prevention of endophthalmitis. The goal of a topical anti-infective is to reduce bacteria on the ocular surface as quickly and safely as possible in order to lessen the likelihood of bacteria entering the eye during surgery or through a poorly sealed wound.
Fourth-generation fluoroquinolones, which target both bacterial topoisomerase IV and DNA gyrase and include besifloxacin, gatifloxacin, and moxifloxacin, have emerged in recent years as the most commonly used anti-infective agents during cataract surgery,[7] although their perioperative use is an off-label indication. These drugs have come to the forefront for their penetration, broad-spectrum microbiological activity, minimal toxicity to ocular tissues, and low potential for bacterial resistance. When fluoroquinolones are used to prevent endophthalmitis, they are typically administered a few days before surgery and are continued for 1-2 weeks, but dosing can vary from surgeon to surgeon.
Several studies have compared both the coverage and penetration of the earlier and later generations of fluoroquinolones, demonstrating broader coverage and deeper penetration among the fourth-generation fluoroquinolones. An in vitro study compared the susceptibility patterns and minimum inhibitory concentration potencies of moxifloxacin and gatifloxacin with the earlier-generation levofloxacin, ciprofloxacin, and ofloxacin among 93 isolates of bacterial endophthalmitis.[8] Fourth-generation fluoroquinolones covered bacterial resistance compared with second- and third-generation fluoroquinolones, were more potent against gram-positive bacteria, and were equally potent for gram-negative bacteria. Studies assessing corneal penetration among the fourth-generation fluoroquinolones found generally higher levels of moxifloxacin in the aqueous humor after topical administration, while other studies have suggested that gatifloxacin may eradicate bacteria more quickly.[9-12] None of the studies have shown any significant side effects from use of fluoroquinolones.
Recent changes to formulations have been developed to allow drugs to remain longer on the cornea and conjunctiva as a way to increase the amount of time for penetration into the aqueous humor and other target tissues and, therefore, to presumably increase the rate at which bacteria can be eliminated. Besifloxacin is formulated with DuraSite® (InSite Vision; Alameda, California), a synthetic polymer designed to extend the residence time on the cornea. In addition, a new formulation of moxifloxacin, containing an enhanced xanthan-gum delivery system, is anticipated in early 2011. Clinical studies comparing the current and new formulations of moxifloxacin demonstrated 1.8-fold higher peak levels of conjunctival moxifloxacin concentrations and larger total tissue exposure with the new formulation compared with the current one.[13]
Resistance to antibiotics is a general concern with use of antibiotics. So far, resistance to the fourth-generation fluoroquinolones has been uncommon[14] and there is some thought that the increased concentration and residence time of the new fluoroquinolones will help prevent resistance. Besifloxacin, which is the most recent addition to the fourth-generation fluoroquinolone class, was specifically developed for ophthalmic use and has no previous systemic use, which may make it less susceptible to bacterial resistance.
Patient adherence is also crucial to a successful outcome. Attempts have been made to remove adherence as a barrier to outcomes by using intracameral antibiotics during surgery. A large European study demonstrated that the risk for endophthalmitis after cataract surgery can be significantly reduced with the administration of an intracameral injection of cefuroxime following each procedure.[15] This practice, however, has not been widely adopted in the United States because of concerns over methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonads, both of which lack sensitivity to cefuroxime. There have been studies of moxifloxacin used intracamerally,[16] but this practice has not been widely adopted.
Although improved surgical methods, such as micro-incisions, have lessened its occurrence, inflammation nevertheless remains a risk with any surgery. Inflammation after cataract surgery begins with tissue injury when the surgical incision is made. Even a small degree of surgical trauma can alter the blood-aqueous barrier, resulting in protein leakage, cellular reaction, and inflammation in the aqueous humor. Uncontrolled inflammation, even low-grade, can lead to conditions such as CME and permanent vision loss. Certain factors, such as diabetes, use of tamsulosin, or history of iritis; and situations, such as dense cataracts or extended phaco time, may increase the risk for inflammation following surgery, but often there is no way to predict who is at risk.[17,18] As such, prophylactic measures are needed.
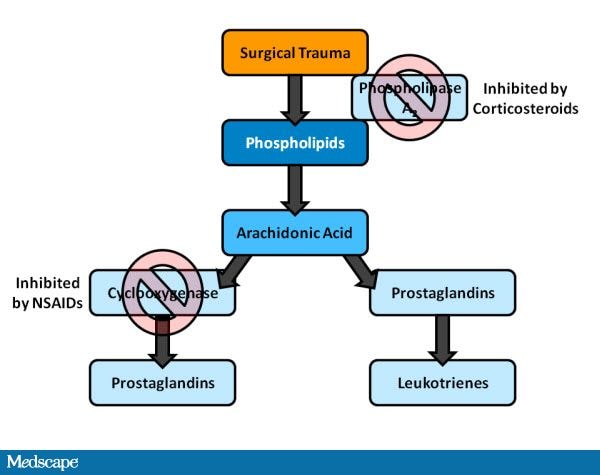
Steroids have been the cornerstone of therapy for controlling inflammation after cataract surgery for over half a century, and their utility has grown as modifications have been made to make them more efficacious, tolerable, and safe. More recently, it has been found that NSAIDs may complement the action of steroids by acting at a different and synergistic place in the inflammatory cascade (Figure). Steroids reduce prostaglandin and leukotriene production by inhibiting the enzyme phospholipase A-2 early in the inflammatory cascade. This early blockage of inflammatory mediators is thought to contribute to the broad anti-inflammatory effects of steroids. Alternately, NSAIDs block cyclooxygenase (COX) pathways and act nonselectively against both COX-1 and COX-2 enzymes. Therefore, NSAIDs inhibit the formation of prostaglandins and subsequent products in the metabolic pathway. This activity, along with numerous studies regarding the concurrent use of steroids and NSAIDs postoperatively, suggest that together, steroids and NSAIDs have a synergistic effect on inflammation.[19] It has become common to employ these agents prophylactically.
Figure 1. Corticosteroid and NSAID effect on the inflammatory cascade.
From Cervantes-Coste G, et al.[21]
NSAIDs were used initially for their ability to prevent intraoperative pupillary miosis and control intraoperative and postoperative pain, both of which are indications approved by the US Food and Drug Administration. The off-label use of NSAIDs to prevent CME has grown considerably over the past several years as several large clinical trials have demonstrated the clinical effectiveness of NSAIDs in the prevention of CME (Table).[19-24]
Table 1. Clinical Trials Assessing Effectiveness of NSAID Prophylaxis of CME
| NSAID | Author, year | N | Study design/comments | Results |
| Nepafenac 0.1% | Wolf,[20] 2007 | 450 | Prophylaxis of visually significant CME (suboptimal BCVA + CME on OCT); nepafenac 0.1% + prednisolone vs prednisolone alone | Decreased incidence of CME (P = .0354) among the group receiving nepafenac 0.1% + prednisolone |
| Cervantes,[21] 2009 | 60 | Prophylaxis of CME; Nepafenac 0.1% + tobramycin-dexamethasone vs tobramycin-dexamethasone alone | Patients in the nepafenac + tobramycin-dexamethasone group had less fluid, as measured by total macular volume (P < .001) | |
| Bromfenac 0.09% | Endo,[22] 2010 | 62 | Prophylaxis of CME as measured by OCT; bromfenac 0.09% vs a steroidal solution | Indications of inflammation, anterior chamber flare, and average perifoveal thickness were significantly reduced at 4 weeks (P = .0009, P < .0001, respectively) and 6 weeks (P = .005, P < .0001) in the bromfenac group |
| Ketorolac 0.4% | Wittpenn,[19] 2008 | 546 | Prophylaxis of clinically apparent CME (slit lamp + OCT); ketorolac 0.4% + prednisolone vs prednisolone alone | Patients in the ketorolac + prednisolone group had reduced incidence of clinically apparent CME (P = .032) and CME based on OCT scan (P = .018) |
| Donnenfeld,[23] 2006 | 100 | Prophylaxis of clinically significant CME (20/30 BCVA + OCT); ketorolac 0.4% preop 3d, 1d, 1hr, or placebo | Nonsignificant decreases in CME and some significant decreases in inflammation among the ketorolac groups | |
| Ketorolac 0.5% | Almeida,[24] 2008 | 106 | Prophylaxis of CME as measured by OCT; ketorolac 0.5% + prednisolone vs prednisolone alone | Total macular volume decreased in the ketorolac group (P = .009) |
BCVA = best corrected visual acuity; CME = cystoid macular edema; OCT = optical coherence tomography
As with anti-infective agents, drug potency and the ability to penetrate into the anterior and posterior chambers, where CME occurs, are both important factors in considering NSAID therapy. Recent research has looked into modifications, such as prodrug formulations, changes in pH, or additions of thickening agents, to help enhance the effectiveness of NSAIDs. A prodrug formulation, in which a reservoir of the parent drug is created in the anterior chamber, has been shown to have rapid permeability and prolonged duration.[25] Similarly, broad COX inhibition is important in NSAID choice. Some NSAIDs demonstrate greater COX-1 inhibition, while others are more effective at reducing COX-2. In one recently published study, aqueous humor concentrations and COX inhibitory activities of nepafenac, amfenac, ketorolac, and bromfenac after topical ocular administration were assessed.[26] The prodrug, nepafenac, had the shortest time to peak concentration and the greatest peak aqueous humor concentration (Cmax).The combined areas under the curve (AUCs) of nepafenac and amfenac were the highest of all drugs tested (P < .05). Ketorolac was the most potent COX-1 inhibitor and amfenac the most potent COX-2 inhibitor.
NSAIDs, like fluoroquinolones, are often started prior to surgery but are usually continued for a longer period of time postoperatively. This ranges from 1 to 2 months after surgery and is often at various dosing frequencies (usually dependent on the indication or surgeon preference). Several years ago, there was some debate regarding the utility of NSAIDs in the perioperative setting, but their safety, utility in reducing inflammation and pain, and demonstrated effectiveness at treating and preventing CME has made their use common and widely accepted.
Corticosteroids are classified as either "potent/strong" or "weak/soft" on the basis of their clinical efficacy and bioavailability. Until recently, the most widely used ophthalmic corticosteroids were loteprednol etabonate 0.5%, a soft steroid, and prednisolone acetate 1%, a strong steroid, both of which are topical ophthalmic suspensions. Prednisolone acetate is the steroid used most often in a cataract surgery perioperative regimen. A new strong steroid, the first in the past 35 years, was recently developed: Difluprednate 0.15%, a novel difluorinated prednisolone derivative, was found to be as effective at controlling inflammation in patients with anterior uveitis when dosed half as often as prednisolone acetate.[27] It is formulated as an emulsion rather than a suspension, which results in a consistent dosage and may help it to better penetrate the cornea.[28,29]
In the past, steroids were usually administered after surgery at various dosing frequencies and for different lengths of time. More recently, it has become common to dose steroids similar to NSAIDS, starting before surgery and continuing for 1-2 months afterwards. Depending on the level of inflammation and the potency of the steroid, dosing frequency can range from once a day to every 2 hours. The most important aspect of steroid dosing is the gradual tapering of the drug, which is done to avoid steroid withdrawal or the return of inflammation.
Steroids have side effects that must be monitored and managed. The most common are a rise in intraocular pressure (IOP) and cataract formation, although the latter is often associated with more long-term steroid use. The exact pathophysiology of the rise in IOP due to topical steroid use is still somewhat unknown. Not all patients taking topical steroids develop elevated IOP, but some known risk factors include preexisting primary open-angle glaucoma, a family history of glaucoma, high myopia, diabetes mellitus, and history of connective tissue disease (especially rheumatoid arthritis).
Advances in pharmacology have allowed ophthalmic surgeons to reduce the incidence of inflammation and infection after cataract surgery. Nonetheless, infection and inflammation still occur, and as such, surgeons must stay abreast of the latest developments in ocular therapeutics.
Supported by an independent educational grant from Alcon.