FDA Clears Candela's Vbeam 595 nm Pulsed Dye Laser for the Treatment of Port Wine Stains and Hemangiomas in the Pediatric Population
The new nod expands the indications to include the pediatric population from birth to 21 years of age.
The U.S Food and Drug Administration has cleared candela’s Vbeam family of 595 nm wavelength pulsed dye lasers (PDL) to treat cutaneous capillary malformations, and infantile hemangiomas (IH)/congenital hemangiomas in the pediatric population (from birth – 21 years of age).
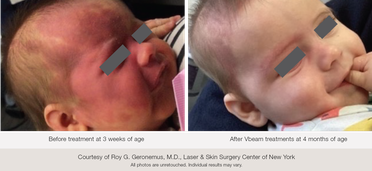
"I am fortunate to be one of the original investigators in the use of the pulsed dye laser for cutaneous vascular lesions close to forty years ago," says Roy G. Geronemus, M.D., Director of the Laser & Skin Surgery Center of New York, in a news release. He provided a recommendation letter to the FDA for clearance of Candela's Vbeam Family of 595 nm PDLs in the pediatric population. "Since that time, the focus of my clinical research and clinical practice has been the treatment of port wine stains and hemangiomas in infants and children. My most recent study of 197 infants with port wine stains under the age of 1 year showed dramatic clearing and the highest incidence of clearing to date."
Port wine stains affect one in every 300 infants, and over time usually get darker and thicker, making early onset treatment critical for optimal results. Early treatment of PWS during childhood leads to the best clinical response. The incidence of His in neonatal patients (under 28 days of age) varies, with a reported rate ranging from 1.1 to 2.6% . It is also believed that hemangiomas grow rapidly during the first months of life. The Vbeam 595 nm PDL stops hemangioma growth, reduces the lesion, and accelerates hemangioma regression, especially in the superficial IH, without serious side effects.
In 33 peer-reviewed published studies, the Vbeam 595 nm PDL was proven to be gentle enough for use in >6,000 pediatric patients with PWS/hemangiomas, while powerful enough to deliver effective and durable results, Candela reports.
The Vbeam 595 nm PDL incorporates the optimal wavelength to penetrate through the target vessels, optimal pulse durations for heating of larger caliber blood vessels, and epidermal protection with its dynamic cooling device (DCD).
Vbeam 595 nm PDL treatment during infancy can be done without general anesthesia, which offers a safety advantage over repeated treatments performed under general anesthesia, and it can maximize the likelihood of clearance with frequent but safe treatment sessions. The Vbeam 595 nm PDL has been chosen by eight of 10 of the top U.S. children's hospitals and dermatologists worldwide, Candela reports.
"The new Vbeam 595 nm PDL clearance adds to the already impressive capabilities of this platform. Candela continues to invest not only in clinical research that will allow physicians to give the necessary care to their patients, but also in our philanthropic partnership with the Vascular Birthmark Foundation. With decades of proven results with the Vbeam PDL, Candela is dedicated to advancing clinical care, and this one-of-a-kind clearance is a big step forward in our mission to improve patients' lives," adds Geoff Crouse, CEO of Candela.

Facebook Comments