
FDA has scheduled the first advisory committee meetings of 2022. One meeting of the Oncologic Drugs Advisory Committee is set for February 10 to discuss a new application for the proposed treatment of Non-Small Cell Lung Cancer (NSCLC. And a meeting of the Anesthetic and Analgesic Drug Products Advisory Committee is meeting jointly with the Drug Safety and Risk Management Advisory Committee on February 15 to consider an NDA in the pain category . But before heading into 2022, it is perhaps time to take stock of AdComms and what they did or did not do during 2021. Fewer Meetings. First and foremost is that as noted earlier this year (See August 5, 2021 FDA Adcomms – Is FDA Getting Less Advice?), there were fewer 2021 meetings held to consider new drug applications than in years gone by. And it isn’t even close. In all there were 20 advisory committee meetings scheduled by FDA this year, but only half of those involved deliberations regarding an application for a new medicine. In fact, the number of AdComms to consider new products was the lowest it has been in a decade, less than one-third what it was ten years prior.
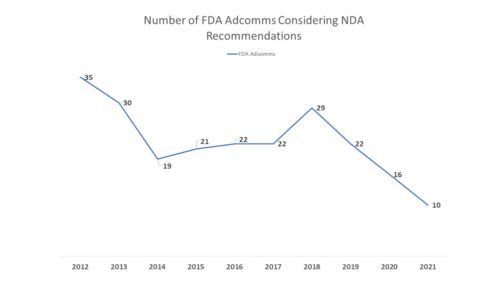
Of note, this tally does not include meetings of the Vaccines and Related Biologic Products Advisory Committee held to consider a recommendation for COVID-19 vaccines, but includes only drugs and biologics.
- Of the ten 2021 meetings, one of them was to consider a supplemental application in oncology – a new indication for Keytruda;
- One of the 2021 meetings was consider approval under Emergency Use Authorization (EUA) for use of molnupiravir to treat mild to moderate COVID-19 at risk for progression;
- While there have been many new oncology drugs to come before FDA this year, only two had advisory committee meetings – the sBLA for Keytruda and a BLA for retifanlimab for anal carcionoma;
- The number of meetings represents a fairly sharp decline in the number of times FDA has gone to advisory committees for input, though the number of novel drug approvals at the agency this year hit a robust 50 for the year. In fact, the decrease in the number of AdComms does not appear to have a correlation to the number of new molecular entities being approved by the agency.
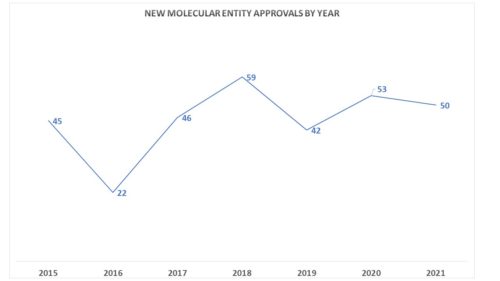
One might be inclined to think that the decline in AdComms is due to the pandemic due to the the complications inherent in staging a meeting under these circumstances. But in fact, FDA has been holding virtual meetings for a long time now. Rather, it is possible that the agency is seeking the input of advisory committees because it is more engaged in reviewing new applications that have priority review status, which is a more interactive effort between the agency and the sponsor.
FDA Outcomes.
- Of the ten AdComm meetings considering new product approvals, companies got a recommendation for approval in half of them, two of them in diabetes. FDA has acted on seven of these recommendations;
- Neither of the two oncology filings got a recommendation for approval.
- Most of the votes taken by the committees were quite lopsided, with only a few of them being close splits, one of which was the vote on molnupiravir for the treatment of COVID-19 (13-10 vote recommending approval).
- So far this year (and unlike last year) there have been no instances where FDA has gone against the advice of a committee. Last year, FDA acted against the recommendations of the committees four times – twice to approve in the face of a recommendation against approval and twice to decline approval where there was a recommendation for approval.
Taking Care of AdComm Vacancies. And speaking of divergent outcomes – one of the most notable occurrences in 2020 was the number of resignations that occurred in the wake of FDA’s decision to approve Aduhelm for Alzheimer’s Disease after an overwhelming committee vote recommending the agency decline to approve. While a count last month showed many vacancies, now FDA is listing very few. Here is where FDA currently has vacancies:
- Bone, Reproductive and Urologic Drugs Advisory Committee – 4 vacancies
- Gastrointestinal Drugs Advisory Committee – 1 vacancy
- Pharmaceutical Science and Clinical Pharmacology Advisory Committee – 4 vacancies
- Pharmaceutical Compounding Advisory Committee – 1 vacancy
In coming days, we will be doing more look-back’s at FDA activities during 2021.

