Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge
Abstract
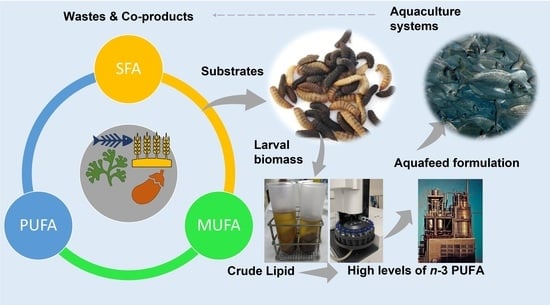
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria and Data Extraction
2.3. Statistical Analysis
3. Results & Discussion
3.1. General Framework
3.2. Lipid Profile of Hermetia illucens Larvae-From Quantitative to Qualitative Approaches
3.3. Manipulation of the Lipid Content of Hermetia illucens for Marine Aquafeeds Formulation
4. Conclusions and New Research Pathways
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ameixa, O.M.C.C.; Soares, A.O.; Soares, A.M.V.M.; Lillebø, A.I. Ecosystem services provided by the little things that run the world. Sel. Stud. Biodivers. 2018, 5, 267–302. [Google Scholar]
- Ameixa, O.M.C.C.; Duarte, P.M.; Rodrigues, D.P. Insects, Food Security and Sustainable Aquaculture. In Zero Hunger; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–11. ISBN 978-3-319-69626-3. [Google Scholar]
- Freccia, A.; Sergio Bee Tubin, J.; Nishioka Rombenso, A.; Gustavo Coelho Emerenciano, M. Insects in Aquaculture Nutrition: An Emerging Eco-Friendly Approach or Commercial Reality? In Emerging Technologies, Environment and Research for Sustainable Aquaculture; Lu, Q., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83881-199-0. [Google Scholar]
- Calvert, C.C.; Martin, R.D.; Morgan, N.O. Housefly pupae as food for poultry. J. Econ. Entomol. 1969, 4, 938–939. [Google Scholar] [CrossRef]
- Hale, O.M. Dried Hermetia illucens larvae (Diptera: Stratiomyidae) as a feed additive for poultry. J. Georg. Entomol. Soc. 1973, 9, 100. [Google Scholar]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Duarte, P.; Maciel, E.; Pinho, M.; Domingues, R.; Calado, R.; Lillebo, A.; Ameixa, O. Omega-3 on the fly: Long-legged fly Machaerium maritimae as a potential source of eicosapentaenoic acid for aquafeeds. J. Insects Food Feed 2021. [Google Scholar] [CrossRef]
- Daniso, E.; Tulli, F.; Cardinaletti, G.; Cerri, R.; Tibaldi, E. European Commission Commision Regulation 2017/893... as regards to the provisions on processed animal proteins. Off. J. Eur. Union 2017, 245, 2373–2381. [Google Scholar]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Anses the Use of Insects as Food and Feed and the Review of Scientific Knowledge on the Health Risks Related to the Consumption of Insects. In Proceedings of the ANSES Opinion. 2015. Available online: https://www.anses.fr/en/system/files/BIORISK2014sa0153EN.pdf (accessed on 14 January 2022).
- Rumpold, B.; Schlüter, O. Nutrient composition of insects and their potential application in food and feed in Europe. Food Chain 2014, 4, 129–139. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout Oncorhynchus mykiss. Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.-J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.-L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable use of Hermetia illucens insect biomass for feed and food: Attributional and consequential life cycle assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Glencross, B.D.; Baily, J.; Berntssen, M.H.G.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2020, 12, 703–758. [Google Scholar] [CrossRef] [Green Version]
- Craig, S.; Helfrich, L.A. Understanding Fish Nutrition, Feeds, and Feeding. Virginia Coop. Ext. 2002, 9, 1–18. [Google Scholar]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Finke, M.D.; Oonincx, D.G.A.B. Nutriënt content of insects. In Insects as Food and Feed: From Production to Consumption; Tomberlin, A., van, H.J.K., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 290–316. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Genetically modified plants are an alternative to oily fish for providing n-3 polyunsaturated fatty acids in the human diet: A summary of the findings of a Biotechnology and Biological Sciences Research Council funded project. Nutr. Bull. 2021, 46, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.A.; Usher, S.; Haslam, R.P.; Ruiz-Lopez, N.; Sayanova, O. Transgenic plants as a sustainable, terrestrial source of fish oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.; Baeshen, M.; Moussa, T.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.; Cancela, M.L.; Carlsson, J.; Carvalho, M.; Cegłowska, M.; Chirivella-Martorell, J.; Cueto, M.; et al. The Essentials of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Ji, X.-J.; Ledesma-Amaro, R. Microbial lipid biotechnology to produce polyunsaturated fatty acids. Trends Biotechnol. 2020, 38, 832–834. [Google Scholar] [CrossRef]
- Francuski, L.; Beukeboom, L.W. Insects in production—An introduction. Entomol. Exp. Appl. 2020, 168, 422–431. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquac. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. Metaboanalystr 3.0: Toward an optimized workflow for global metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Gan, S.L.; Leong, S.Y.; Chin, K.S. Sulphonated rice husk biochar for in-situ methanolysis of fatty acid methyl ester from H. illucens. AIP Conf. Proc. 2018, 2020, 020059. [Google Scholar]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Leong, S.Y.; Chong, S.S.; Chin, K.S. Biodiesel derive bio-oil of Hermetia illucens pre-pupae catalysed by sulphonated biochar. E3S Web Conf. 2018, 34, 02004. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Xiong, H.; Wang, W.; Duan, X.; Yang, T.; Wu, C.; Yang, F.; Wang, T.; Wang, C. A facile and mild one-pot process for direct extraction of lipids from wet energy insects of black soldier fly larvae. Renew. Energy 2020, 147, 584–593. [Google Scholar] [CrossRef]
- Bastrakov, A.I.; Zagorinsky, A.A.; Kozlova, A.A.; Ushakova, N.A. Bioconversion of solid organic wastes by maggots of black soldier flies (Hermetia illucens). In Life Chemistry Research: Biological Systems; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781498700009. [Google Scholar]
- Leong, S.Y.; Kutty, S.R.M.; Tan, C.K.; Tey, L.H. Comparative study on the effect of organic waste on lauric acid produced by Hermetia illucens larvae via bioconversion. J. Eng. Sci. Technol. 2015, 8, 52–63. [Google Scholar]
- Beskin, K.V.; Holcomb, C.D.; Cammack, J.A.; Crippen, T.L.; Knap, A.H.; Sweet, S.T.; Tomberlin, J.K. Larval digestion of different manure types by the black soldier fly (Diptera: Stratiomyidae) impacts associated volatile emissions. Waste Manag. 2018, 74, 213–220. [Google Scholar] [CrossRef]
- Chun, C.Y.; Yoong, L.S.; Kim, L.P.; Hock, T.L.; Ling, L.J. Comparison of Hermetia illucens larvae and pre-pupae as potential aqua feed derived from the biotransformation of organic waste. AIP Conf. Proc. 2019, 2157, 020008. [Google Scholar]
- Liu, T.; Awasthi, M.K.; Chen, H.; Duan, Y.; Awasthi, S.K.; Zhang, Z. Performance of black soldier fly larvae (Diptera: Stratiomyidae) for manure composting and production of cleaner compost. J. Environ. Manag. 2019, 251, 109593. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; Sánchez-Muros, M.J.; Rincón, M.Á.; Rodriguez-Rodriguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.L. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Riolo, P.; Isidoro, N.; Conti, C.; Zarantoniello, M.; et al. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020, 259, 114309. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly ( Hermetia illucens ) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of vegetable and fruit substrates as potential rearing media for Hermetia illucens (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life cycle assessment of edible insects for food protein: A review. Agron. Sustain. Dev. 2016, 36, 57. [Google Scholar] [CrossRef] [Green Version]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2018, 11, 1080–1103. [Google Scholar] [CrossRef]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable Resources from insects: Exploitation, properties, and refining of fat obtained by cold-pressing from Hermetia illucens (Black Soldier Fly) Larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- Adam Mariod, A. Insect oil and protein: Biochemistry, food and other uses: Review. Agric. Sci. 2013, 4, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Stanley-Samuelson, D.W.; Dadd, R.H. Long-chain polyunsaturated fatty acids: Patterns of occurrence in insects. Insect Biochem. 1983, 13, 549–558. [Google Scholar] [CrossRef]
- Singh, A.; Kumari, K. An inclusive approach for organic waste treatment and valorisation using Black Soldier Fly larvae: A review. J. Environ. Manag. 2019, 251, 109569. [Google Scholar] [CrossRef] [PubMed]
- Abduh, M.Y.; Jamilah, M.; Istiandari, P.; Manurung, R.; Muhammad, C.; Abduh, Y. Bioconversion of rubber seeds to produce protein and oil-rich biomass using black soldier fly larva assisted by microbes. J. Entomol. Zool. Stud. 2017, 5, 591–597. [Google Scholar]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Tran, G.; Heuzé, V.; Makkar, H.P.S. Insects in fish diets. Anim. Front. 2015, 5, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2019, 27, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Moula, N.; Scippo, M.-L.; Douny, C.; Degand, G.; Dawans, E.; Cabaraux, J.-F.; Hornick, J.-L.; Medigo, R.C.; Leroy, P.; Francis, F.; et al. Performances of local poultry breed fed black soldier fly larvae reared on horse manure. Anim. Nutr. 2018, 4, 73–78. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Nadia, M.H.; Syaripudin; Manurung, R.; Putra, R.E. Factors affecting the bioconversion of Philippine tung seed by black soldier fly larvae for the production of protein and oil-rich biomass. J. Asia. Pac. Entomol. 2018, 21, 836–842. [Google Scholar] [CrossRef]
- Vargas, A.; Randazzo, B.; Riolo, P.; Truzzi, C.; Gioacchini, G.; Giorgini, E.; Loreto, N.; Ruschioni, S.; Zarantoniello, M.; Antonucci, M.; et al. Rearing zebrafish on Black Soldier Fly (Hermetia illucens): Biometric, histological, spectroscopic, biochemical, and molecular implications. Zebrafish 2018, 15, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Woods, M.J.; van Emmenes, L.; Pieterse, E.; Hoffman, L.C.; Dalle Zotte, A. Hermetia illucens larvae leared on different substrates in broiler quail diets: Effect on physicochemical and sensory quality of the quail meat. Animals 2019, 9, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.-W.; Mohd-Noor, S.-N.; Wong, C.-Y.; Lam, M.-K.; Goh, P.-S.; Beniers, J.J.A.; Oh, W.-D.; Jumbri, K.; Ghani, N.A. Palatability of black soldier fly larvae in valorizing mixed waste coconut endosperm and soybean curd residue into larval lipid and protein sources. J. Environ. Manag. 2019, 231, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.; Huis, A.; van Loon, J. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Alifian, M.D.; Sholikin, M.M.; Evvyernie, D. Nahrowi Potential fatty acid composition of Hermetia illucens oil reared on different substrates. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 62002. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M. Characteristic of Hermetia illucens fatty acid and that of the fatty acid methyl ester synthesize based on upcycling of perishable waste. Waste Biomass Valorization 2020, 11, 5607–5614. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Ke, J.; Chen, J.; Holtzapple, M.T.; Tomberlin, J.K.; Chen, H.; Zhang, J.; Li, Q. Production of biodiesel from CO2 and organic wastes by fermentation and black soldier fly. Renew. Energy 2019, 149, 1174–1181. [Google Scholar] [CrossRef]
- Alipour, N.; Vinnerås, B.; Gouanvé, F.; Espuche, E.; Hedenqvist, M. A protein-based material from a new approach using whole defatted larvae, and its interaction with moisture. Polymers 2019, 11, 287. [Google Scholar] [CrossRef] [Green Version]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Xu, X.; Ji, H.; Belghit, I.; Liland, N.S.; Wu, W.; Li, X. Effects of black soldier fly oil rich in n-3 HUFA on growth performance, metabolism and health response of juvenile mirror carp (Cyprinus carpio var. specularis). Aquaculture 2021, 533, 736144. [Google Scholar] [CrossRef]
- El-Dakar, M.A.; Ramzy, R.R.; Ji, H.; Plath, M. Bioaccumulation of residual omega-3 fatty acids from industrial Schizochytrium microalgal waste using black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2020, 268, 122288. [Google Scholar] [CrossRef]
- Agbohessou, P.S.; Mandiki, S.N.M.; Gougbédji, A.; Megido, R.C.; Hossain, M.S.; De Jaeger, P.; Larondelle, Y.; Francis, F.; Lalèyè, P.A.; Kestemont, P. Total replacement of fish meal by enriched-fatty acid Hermetia illucens meal did not substantially affect growth parameters or innate immune status and improved whole body biochemical quality of Nile tilapia juveniles. Aquac. Nutr. 2021, 27, 880–896. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Riudavets, J.; del Arco, L.; Castellari, M. Impact of diets including agro-industrial by-products on the fatty acid and sterol profiles of larvae biomass from Ephestia kuehniella, Tenebrio molitor and Hermetia illucens. Insects 2021, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Enrichment in specific fatty acids profile of Tenebrio molitor and Hermetia illucens larvae through feeding. Future Foods 2021, 3, 100016. [Google Scholar] [CrossRef]
- Somroo, A.A.; ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The effects of diet formulation on the yield, proximate composition, and fatty acid profile of the Black Soldier Fly (Hermetia illucens L.) prepupae intended for animal feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhu, S.; Liu, D.; Guo, X.; Ye, Z. Effects of stocking density of the white shrimp Litopenaeus vannamei (Boone) on immunities, antioxidant status, and resistance against Vibrio harveyi in a biofloc system. Fish Shellfish Immunol. 2017, 67, 19–26. [Google Scholar] [CrossRef]
- Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects Future Prospects for Food and Feed Security; Wageningen UR, Ed.; FAO Forest; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 171, ISBN 978-92-5-107595-1. [Google Scholar]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect rearing: Potential, challenges, and circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]





| Keywords | Web of Science™ | Scopus |
|---|---|---|
| Hermetia illucens + fatty acids | 196 | 214 |
| Black Soldier Fly + fatty acids | 183 | 205 |
| PUFA + Hermetia illucens | 23 | 21 |
| PUFA+ Black Soldier Fly | 23 | 24 |
| n-3 + Hermetia illucens | 32 | 34 |
| n-3 + Black Soldier Fly | 27 | 33 |
| omega 3 + Hermetia illucens | 8 | 10 |
| omega 3 + Black Soldier Fly | 23 | 26 |
| HUFA + Hermetia illucens | 2 | 2 |
| HUFA + Black soldier fly | 2 | 2 |
| Hermetia illucens + fatty acids + review | 18 | 6 |
| Black Soldier Fly + fatty acids + review | 7 | 2 |
| PUFA + Hermetia illucens + review | 0 | 0 |
| n-3 + Hermetia illucens + review | 1 | 0 |
| omega 3 + Hermetia illucens + review | 1 | 2 |
| HUFA + Hermetia illucens+ review | 0 | 0 |
| Total of publications | 546 | 581 |
| Total of publications considered after removing duplicates | 392 | |
| Total of publications considered after selection criteria | 47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, D.P.; Ameixa, O.M.C.C.; Vázquez, J.A.; Calado, R. Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge. Sustainability 2022, 14, 6472. https://doi.org/10.3390/su14116472
Rodrigues DP, Ameixa OMCC, Vázquez JA, Calado R. Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge. Sustainability. 2022; 14(11):6472. https://doi.org/10.3390/su14116472
Chicago/Turabian StyleRodrigues, Daniela P., Olga M. C. C. Ameixa, José Antonio Vázquez, and Ricardo Calado. 2022. "Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge" Sustainability 14, no. 11: 6472. https://doi.org/10.3390/su14116472