From 2009 to 2016, data from the National Health and Nutrition Examination Survey (NHANES) collected from nearly 9,000 people revealed that only 12% of, or 1 in 8, Americans were considered “metabolically healthy,” a statistic circulating the media ever since.
“Metabolic health” has become a health goal many are trying to achieve, which we highly commend. The problem is, “metabolic health” lacks a clear definition. Intuitively, finding the right inputs is challenging without knowing the exact output. So, while directionally we have a good sense of what better metabolic health may look like, the specifics of what’s optimal are blurry.
If you are confused by what “metabolic health” means, rest assured, scientists and researchers are still trying to figure it out. Like “metabolic flexibility”, it is a somewhat nebulous term often thrown around. Even within the literature, the term lacks a clear and concise definition.
What is metabolic health, and why is it important?
Metabolic health is often defined as the absence of metabolic syndrome, which isn’t exactly how we would define it. For reference, metabolic syndrome is diagnosed when a patient has 3 out of 5 of the following: (1) waist circumference greater than 40 inches in men and 35 inches in women (I.e., abdominal obesity), (2) triglycerides at or above 150 mg/dL (3) HDL cholesterol of less than 50 mg/dL in women and 40 mg/dL in men (4) systolic blood pressure of 130 mmHg or greater or diastolic blood pressure of 85 mmHg or greater without medication (5) fasting blood sugar levels of 100 mg/dL or more. That means in some studies, the criteria for metabolic health is a far cry from what we think should be considered optimal. The absence of a disease doesn’t inherently imply “health,” and having less than three of these components does not mean being metabolically healthy. Needless to say, it is necessary to apply a bit more rigor to the definition of metabolic health and expand past the components of metabolic syndrome.
We view metabolic health as a spectrum of metabolic flexibility, glycemic control, insulin sensitivity, and inflammation, all of which are not mutually exclusive and common amongst metabolic diseases, including diabetes, cardiovascular disease, certain cancers, and dementia, among others. Thus, maintaining or striving to achieve metabolic health is incredibly important for long-term health and longevity. The good news is that our daily lifestyles strongly influence our metabolism and, consequently, our metabolic health – this includes how much we eat (eg. CICO), what we eat, macronutrient composition, how often we exercise, type of exercise, our sleep, stress, circadian rhythms, etc., which means that metabolic health is an actionable variable we can optimize, or at least vastly improve.
Without active inputs, maintaining metabolic health appears to be difficult, as both normal weight and obese individuals who are metabolically healthy at baseline transition to a metabolically unhealthy phenotype over time. It is important to identify biomarkers that can help determine the progression of metabolic decline before disease onset. These underlying symptoms (e.g., metabolic inflexibility, glycemic variability, insulin resistance, and inflammation) often occur years before clinical signs of metabolic disorders. However, doctors don’t routinely screen for the markers that may be at the root cause of poor metabolic health. The trend towards poor metabolic health is often correlated with weight gain, but not always. Thus, it is critical that medical professionals and researchers alike agree on a concise definition and specific determinants of optimal metabolic health in order to monitor, prevent, and course-correct poor and/or declining metabolic health.
After a lot of research and Dr. D’Agostino’s experimentation, he has identified five biomarkers beyond the five components of metabolic syndrome previously mentioned, which can provide considerable insight into the state of your metabolic health. These include real time continuous blood glucose monitoring (CGM), blood ketones, breath ketones, fasting insulin, and high sensitivity C-reactive protein (hs-CRP).
Continuous Glucose Monitoring (CGM)
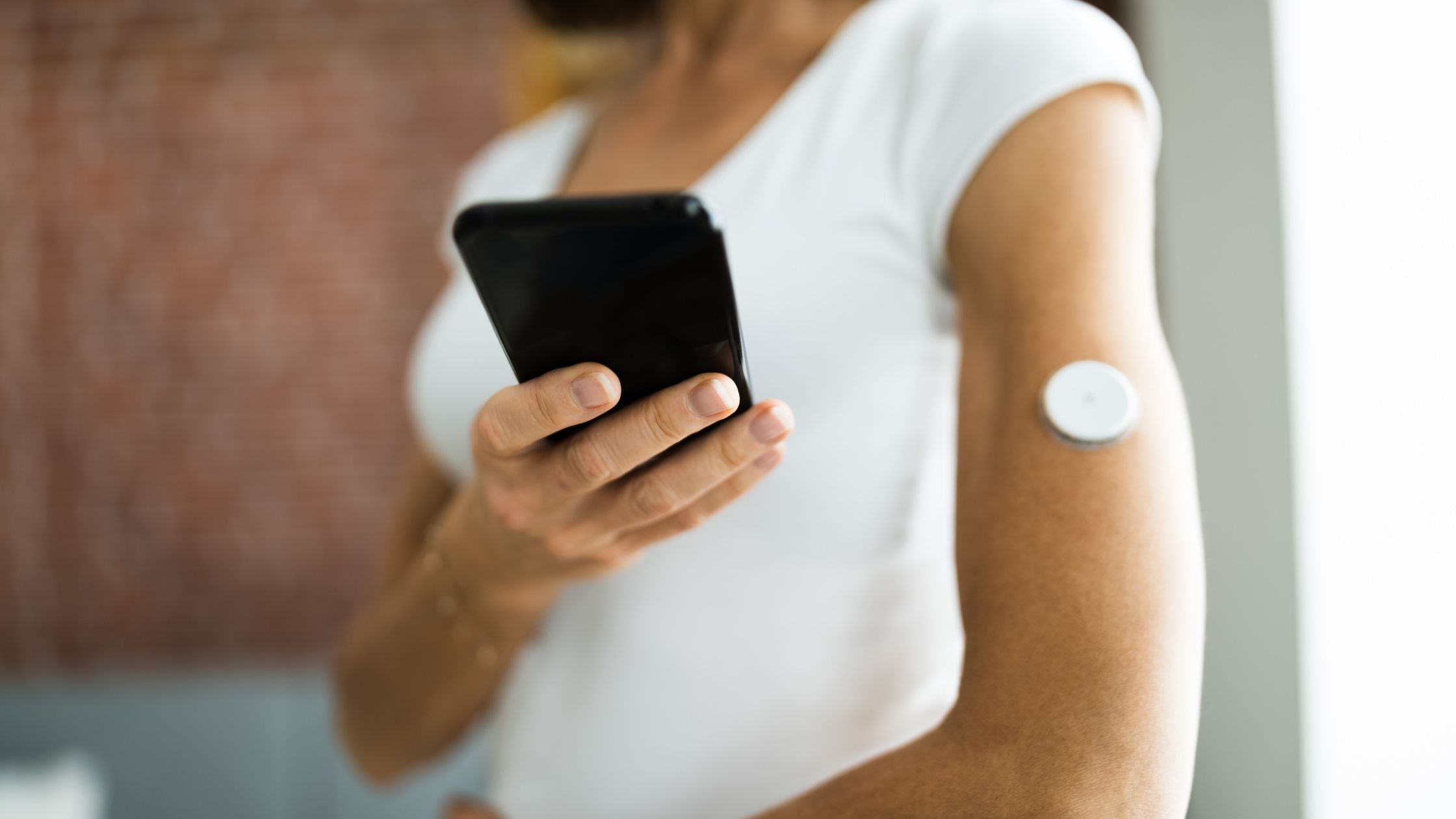
Blood sugar levels play an important role in the onset, identification, and reversal of metabolic dysfunction, as they are intimately related to insulin resistance, inflammation, and of course, glycemic control. Simply put, too much glucose in the bloodstream can lead to metabolic dysfunction. Fasting blood sugar is almost always reported in a typical blood draw by your doctor, which most people get once or twice a year (if that). However, the information received from any single snapshot of blood glucose is limited. In addition, fasting blood sugar levels do not tell us anything about postprandial blood sugar levels (blood sugar after eating), the latter of which is a better representation of HbA1c, a marker of average blood sugar levels.
We can measure blood sugar continuously in real-time using a CGM device. These devices can be incredibly insightful, providing feedback on how our diet, sleep, exercise, stress, and more affect our blood sugar levels. Alternatively, you can measure blood sugar in 15-30 minute intervals using a finger prick device to extend the “snapshot” at any moment in time. Still, there is a definite advantage to measuring blood glucose continuously with a CGM due to providing average blood sugar levels, which is more informative than HbA1c. Most importantly, CGMs can have a powerful influence over our behaviors, and most notably, food choices. You will come to realize the foods that cause unfavorable glycemic excursions, and be able to correlate this with subjective experiences (eg. energy crash). This closed-loop CGM feedback and associated software will lead to better awareness and will motivate changes for better meal portioning and food choices.
What to look for:
- Variability (fluctuations in blood sugar): You want to stay within a target range of ~70-110 mg/dL (~3.9-6.1 mmol/L). Note that if you follow a low carbohydrate ketogenic diet or engage in intermittent fasting, your lower threshold may be more like 55 mg/dL (~3.1 mmol/L). Preventing large fluctuations in blood sugar is important for controlling food intake, body weight, mood, and appetite, directly and indirectly affecting metabolic health.
- Postprandial spikes and rate of rise (how quickly or slowly blood sugar is rising): You want to prevent rapid spikes, and most importantly, if your blood sugar does spike, it should come back down to baseline quickly. Sustained elevation of glucose after consuming carbohydrates is likely a sign of insulin resistance.
- Postprandial dips: If your blood sugar falls below baseline following a spike, this is a sign that your body “overshot” insulin for the amount of glucose or continued to secrete insulin after the meal was digested. The consequence of this is a drop in blood sugar lower than it should and can trigger fatigue, lightheadedness, shakiness and cravings for more carbs.
- Morning elevation of blood glucose is normal for most people. This physiological response has been given the name “dawn effect” and is likely a consequence of the ACTH-induced cortisol awakening response. It is thought that this is an anticipatory response associated with work demands or other psychological stressors can be a contributing factor.
How to improve glycemic control:
- Avoid refined carbohydrates (sugars and flours), especially in liquid form (sugar-sweetened beverages, fruit juices, etc.)
- If you are going to eat carbohydrates, select high-fiber sources (> 20% of total carbs), which will help glycemic response, hunger and microbiome.
- Avoid eating carbohydrates alone. When eating a carb-containing meal, consuming protein, fat and fiber (veggies) before eating carbohydrates (in the same meal) will attenuate the glycemic spike and overall response.
- Time your carbohydrates around exercise. Muscle is our body’s primary “glucose sink,” meaning it’s a place we can safely store glucose without causing metabolic damage. Exercise is the best way to take advantage of this glucose sink by facilitating non-insulin GLUT4-mediated glucose uptake. Thus, by consuming carbohydrates around exercise, we create room for glucose storage, preventing it from circulating in the bloodstream or being stored as fat.
- Avoid eating large meals late at night and close to bedtime (>2 hours before) as this is when we are least insulin sensitive, and therefore are more likely to experience a rise in blood sugar to a given amount of carbohydrates. Late eating is also a sleep disruptor.
- Eat in a condensed eating window. Consuming all caloric intake within an 8-hour eating window vs a 15-hour eating window has been shown to improve overnight and postprandial blood sugar levels.
Recommendations: Levels?
Blood and Breath Ketones
As humans, we rely on two primary fuels: glucose and fat. A healthy metabolism can switch between the two with ease, which defines metabolic flexibility. Blood ketones are one way to identify when fat is being used as our primary source of fuel. This is because when we fast or restrict carbohydrates, our body can only rely on our limited glucose stores for so long go before eventually turning to fat for fuel. During prolonged periods of low glucose availability, the liver converts fats to ketones, releasing them into the blood to serve as an alternative fuel to glucose. So, essentially, when we measure blood ketones, we can see which fuel tank we are pulling from. This can also inform you of your food choices when following a ketogenic diet.
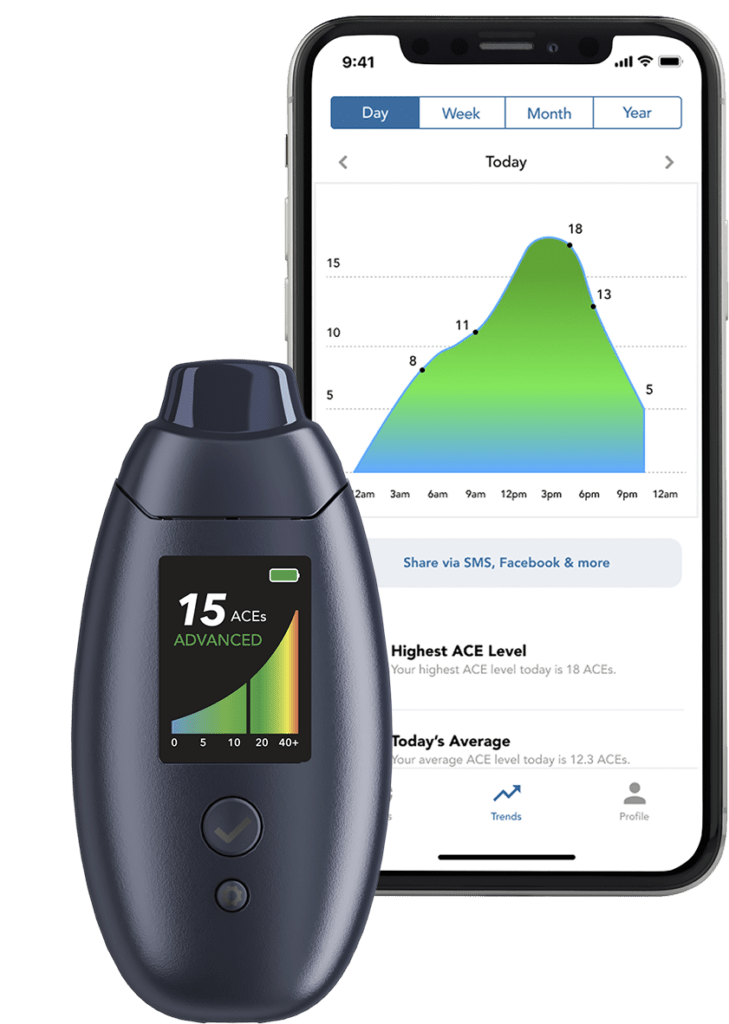
Similar to blood ketones, breath ketones are something we can measure to assess fat-burning and metabolic flexibility. The difference is the circumstances in which they are applied and the type of ketone they are measuring. When we are in a state of ketosis, we produce three primary ketone bodies: acetoacetate (AcAc), beta-hydroxybutyrate (BHB; which we measure when we test blood ketones), and acetone. Acetone is produced by the spontaneous breakdown of AcAc and is a regular molecule found in our breath, but when we are in ketosis, its concentration increases. Acetone is responsible for the “fruity” smell you may experience in a state of ketosis.
What’s neat about breath ketones is that it appears to be a better proxy for fat burning in the fasted state, since during prolonged fasting, BHB is quickly swept up by our cells for fuel, thus lowering the amount in the blood. You can see this for yourself when exercising, blood ketones typically drop (as you are using them for energy), but breath ketones rise (as a better reflection of fat-derived ketones). The utility of measuring your ability to burn fat is a representation of your metabolic flexibility. The more metabolically healthy you are, the easier it should be to make this “metabolic switch.” Since functioning mitochondria are absolutely required to burn fat and ketones efficiently, ketosis (and metabolic flexibility) may also be a marker for mitochondrial health. Indeed, people with poor metabolic health (insulin resistance) also have a reduced capacity to burn fat. Ketosis and fasting can be thought of as “metabolic training” to improve mitochondrial function. Ketosis (elevated ketone levels) is also a marker of low blood sugar since ketones result from low glucose availability and suppression of insulin levels, which may help with improving insulin sensitivity. And lastly, ketones dampen inflammatory signaling and likely protect against oxidative stress, both of which contribute to worse metabolic health and chronic disease.
What to look for:
- What type of ketone readings should people be looking for, for a given intervention?
- The time it takes to enter ketosis during a prolonged fast
- Symptoms associated with the transition into ketosis: if you initially experience extreme discomfort or symptoms of hypoglycemia while fasting or following a ketogenic diet, this may be a sign of poor metabolic flexibility. These symptoms should improve with time as you improve your metabolic health and insulin sensitivity.
- If you measure your ketones and glucose simultaneously, you can calculate your glucose-ketone index (GKI), your glucose divided by your ketones (in mmol/L).. Maintaining a GKI of 1-4 is difficult to do, but is indicative of being in a deep state of nutritional (or fasting) ketosis.
- Breath ketones can be measured with Readout Health’s Biosense. When achieving a state >25 ACEs (or in “HI” state) this is indicative of a very high fat oxidation state.
How to improve metabolic flexibility:
- Metabolic flexibility is the capacity for the organism to adapt fuel oxidation to fuel availability. The inability to tolerate fasting is a good sign you have low metabolic flexibility.
- Engaging in intermittent fasting is the fastest way to establish a caloric deficit to improve it. Periodically emptying your glycogen and “forcing” your body to burn fat is one way to train metabolic flexibility.
- Consider following a ketogenic diet for at least one to six months.
- Exercise in the fasted state (after an overnight fast). Exercising in a low glycogen state can essentially expedite the transition into ketosis.
Fasting Insulin
Insulin wears many hats, but its primary job is managing blood sugar. It is the hormone released in response to elevated blood sugar levels to clear excess glucose out of the blood and shuttle it into our cells. Fasting insulin is an overlooked biomarker we should all be keeping an eye on if we are trying to prevent and/or reverse insulin resistance. Oddly, this is not routinely measured with standard bloodwork, despite a clear advantage to testing fasting insulin compared to fasting glucose. When we measure fasting glucose, we have no idea what’s going on in the background, i.e., how much insulin is needed to keep glucose at that level. So, glucose may look normal, but testing insulin reveals a much clearer picture of metabolic control. The transition from metabolically healthy to metabolically unhealthy is thought to progress under chronically high insulin levels, so catching high insulin before it progresses to high glucose creates a better opportunity for actionable prevention.
What to look for:
- The best method we found to measure insulin (and other cardiometabolic markers) at home is using a ZRT Laboratory blood spot card. (CardioMetabolic Profile Kits measures)– Insulin, HbA1c, TGs, CH, LDL, HDL, VLDL and hsCRP).
- Optimal fasting insulin should be in the 2-6 μIU/mL range.
- When doing prolonged fasting (>48hrs) it may dip on the lower end or slightly below that range and this is normal, and perhaps optimal for extended fasts (eg. fat loss, autophagy, etc.).
High Sensitivity C-Reactive Protein (hsCRP)
You may not be as familiar with hsCRP as you are glucose, ketones, and insulin. hsCRP is a marker of inflammation, as CRP is integral to our immune system, and it is an important biomarker for assessing metabolic health. Note that hsCRP is just a more accurate measure of CRP.
In response to a physical insult, inflammation, or illness, the liver releases CRP into the blood, where it flags any cells or pathogens to be cleared from the blood. Under normal conditions, CRP will rise, do its job, and fall back to normal levels. If the body is chronically inflamed, however, CRP levels remain elevated. As previously mentioned, chronic inflammation is intimately related to metabolic damage and metabolic diseases. High CRP levels are found in those with obesity, heart disease, diabetes, Alzheimer’s disease, and other states of chronic inflammation. Notably, all features of metabolic syndrome are associated with increased levels of CRP. Elevated CRP is also a risk factor and predictor of adverse health outcomes. So, there is high value in keeping track of your hsCRP levels from both a preventative and treatment standpoint.
High hsCRP is also an indicator of leaky gut or gut permeability. Briefly, between the epithelial cells that line the GI tract are “tight junctions,” which regulate what leaves the gut and enters the body. If these tight junctions are impaired, the gut becomes “permeable” to pathogens and potentially harmful compounds that can now cross the gut barrier and “leak” into the bloodstream. In response, the immune system is activated, and hsCRP is released.
What to look for:
- We recommend testing at least once per year when you feel rested and healthy.
- Test hsCRP once a quarter is ideal if you making changes to your diet or working to manage a chronic inflammatory disease process (eg. Cronh’s, arthritis, etc.)
- Keeping hsCRP <3 mg/L is ideal
Routinely measuring these five biomarkers is an excellent way to assess and stay on top of your metabolic health, and their value increases when correlated with other data. Together, these biomarkers can be incredibly informative and can be used to optimize glycemic control, metabolic flexibility, insulin sensitivity, and inflammation. These features of our health are critical for the prevention of chronic disease and increasing longevity.
Written by: Kristi Storoschuk; Edited by: Dominic DAgostino