The MiniMed 770G is significant because it has smartphone connectivity and will be available to patients as young as 2-years-old.
September 1, 2020
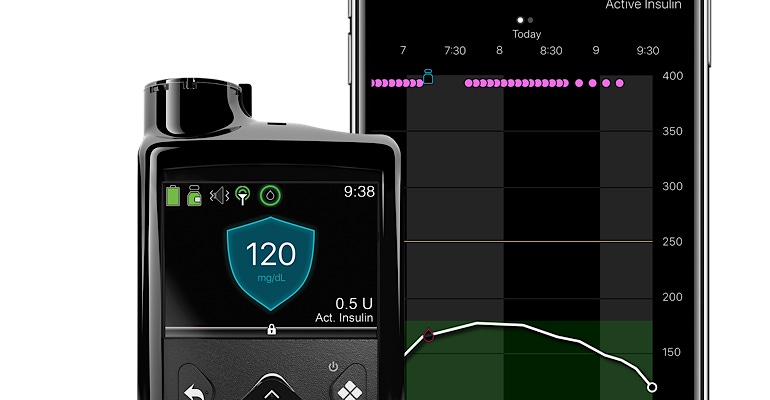
A new approval could help Medtronic cover lost ground in the diabetes market. The Dublin-based company recently received a nod from FDA for the MiniMed 770G hybrid closed-loop system.
The device is significant because it has smartphone connectivity and will be available to patients as young as 2-years-old. Medtronic’s system will enable caregivers and care partners to see user data remotely on their smartphones, with proactive in-app notices sent when sugar levels are out of range.
Medtronic said that a clinical study of the MiniMed 670G system conducted in children two to six years of age showed an improvement in outcomes comparable to those observed in older adolescents and adults, and supported the submission of the MiniMed 770G system. In the study, A1C and Time in Range from 151 children were assessed alongside outcomes from 124 adolescents and adults over two weeks in Manual Mode and three months in SmartGuard Auto Mode (hybrid closed loop algorithm). There were no episodes of severe hypoglycemia or diabetic ketoacidosis, and no serious device-related adverse events while in SmartGuard Auto Mode.
Medtronic set the diabetes management world on fire in 2016 when it won FDA approval for the MiniMed 670G insulin pump, dubbed the artificial pancreas. Despite the landmark approval, the company has struggled with its diabetes offerings.
In Medtronic’s most recent earnings call, CEO Geoff Martha said the firm was missing out on the diabetes market growth.
"Look, we're missing out on the better growth of this market, and nobody at Medtronic is comfortable with this dynamic," Martha said according to a Seeking Alpha transcript of the call. "And we're pushing on several fronts to advance our technology. We're actively increasing both our near and our long-term growth opportunities, through increased organic investment, innovative funding with our recently announced Blackstone partnership, and inorganic activity, highlighted by the announcement earlier this month of our pending acquisition of Companion Medical."
He added, “but make no mistake, we are still very focused on regaining technology leadership in the pump and the sensor market. However, we're also going to meet patients where they are, and provide them with real-time data-guided support. We expect to build a system that combines in pen with our Smart CGM technology, including our neutrino and clue artificial intelligence algorithms. All of this designed to deliver better outcomes and reduce the burden of managing the disease for MDI patients."
About the Author(s)
You May Also Like




