NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Niacin, also known as nicotinic acid and vitamin B3, is a water soluble, essential B vitamin that, when given in high doses, is effective in lowering low density lipoprotein (LDL) cholesterol and raising high density lipoprotein (HDL) cholesterol, which makes this agent of unique value in the therapy of dyslipidemia. Niacin can cause mild-to-moderate serum aminotransferase elevations and high doses and certain formulations of niacin have been linked to clinically apparent, acute liver injury which can be severe as well as fatal.
Background
Niacin (nye" a sin) is a soluble B vitamin and pyridine derivative and an essential dietary element, deficiency of which causes pellagra. The recommended dietary allowance (RDA) of this vitamin is 14 to 16 mg daily in adults, and slightly more for pregnant women (18 mg) and less for children (2 to 12 mg). Niacin given at or around these doses is not associated with significant side effects or liver injury. Niacin is a component of most multivitamin and vitamin B preparations in concentrations close to the minimum daily requirement, which are not effective in lowering lipid levels. Niacin is also found in many herbal mixtures and energy drinks, but generally in low or modest doses.
The doses of niacin used in the therapy of hyperlipidemia are far higher than the RDA and are generally in the range of 1 to 6 grams daily. When given at these doses, niacin has been shown to increase HDL and lower LDL cholesterol levels and to decrease rates of cardiovascular events in high risk individuals. The mechanism of action of niacin in hyperlipidemia is not well understood, but is believed to be related to inhibition of cAMP signaling pathways in adipocytes, which results in decreased release of lipids from fat cells. Niacin was officially approved for use in the United States in 1957 and is still widely used, although its role in management of hyperlipidemia in patients taking statins and other cholesterol lowering agents remains uncertain and controversial. Regular niacin is available in multiple generic forms, under several brand names (including Niacor), in many concentrations as either tablets or capsules from 50 to 1,000 mg each. When used to treat hyperlipidemia, regular niacin is generally referred to as intermediate release [IR] niacin. IR-niacin must be taken several times daily and is associated with a high rate of cutaneous flushing. The recommended dosage for hyperlipidemia is 1 to 6 grams daily, starting at low doses (100 mg three times daily) and increasing at weekly intervals based upon tolerance and effect. Sustained release [SR] formulations of niacins have been developed which are available over-the-counter. SR niacin can be taken once daily and is less likely to cause flushing, but is not approved for use in hyperlipidemia and has been associated with a high rates of hepatotoxicity in some studies. Extended release (ER) capsules and tablets of niacin are available in concentrations ranging from 125 to 1,000 mg, which are approved for use in hyperlipidemia and have not been associated with a higher rate of hepatotoxicity compared to regular niacin. Niacin ER is available by prescription and over-the-counter in generic forms and under several brand names such as Niaspan and Niobid. In recent years the clinical use of niacin has decreased, but currently more than 1 million prescriptions are filled yearly. The recommended daily dosage of niacin ER ranges from 500 to 2,000 mg generally given once daily at bedtime. Niacin is also available in combination with other lipid lowering drugs such as lovastatin (Advicor). Common side effects of niacin include nausea, fatigue, pruritus and flushing; flushing being a major dose-limiting side effect. Less common but potentially severe adverse reactions with long term use include increase risk of serious bleeding, infections, myopathy and hyperglycemia and worsening as well as de novo development of diabetes.
Hepatotoxicity
Niacin in doses above 500 mg daily causes transient, asymptomatic elevations in serum aminotransferase levels in up to 20% of people. The elevations are rarely greater than 3 times the upper limit of the normal range and usually resolve spontaneously even with continuation of the drug. The effect is partially dose related and is more common with doses above 3 g/day. In some patients, there is an overall decrease in serum proteins synthesized by the liver and, in some instances, coagulopathy with an increase in prothrombin time and decline in serum albumin, coagulation factors and apolipoproteins. These changes resolve rapidly upon stopping therapy and may not recur with lower doses.
Niacin can also cause serious hepatotoxicity, but this is uncommon. Significant hepatotoxicity is particularly common with high doses of sustained release niacin. In many cases, the injury becomes apparent after a dose increase or after switching from the regular crystalline to a sustained release form. The pattern is primarily hepatocellular, although cases with a cholestatic pattern have been described. The patients present with jaundice, itching, nausea, vomiting and fatigue. When the injury is the result of switching from the crystalline to the sustained release form, the injury may present acutely within days or a few weeks with a prodromal period of nausea, vomiting and abdominal pain, that is followed by jaundice and pruritus. Early during the injury serum aminotransferase levels are very high and then usually fall rapidly with discontinuation or dose lowering. The clinical phenotype resembles acute hepatic necrosis, suggesting a direct toxic effect. Imaging studies of the liver may reveal areas of hypodensity ("starry sky liver") interpreted as focal fatty infiltration that resolves after stopping the drug. Liver biopsy typically shows varying degrees of centrolobular necrosis with only mild inflammation.
Likelihood score: A[HD] (well known cause of clinically apparent liver injury when given in high doses).
Mechanism of Injury
The mechanism of hepatotoxicity is assumed to be an intrinsic toxic reaction related to high serum levels of niacin that overwhelm the high affinity, low concentration nicotinic acid receptors (that are responsible for the flushing response). The finding that niacin can be restarted at lower doses after an episode of clinically apparent injury indicates that the hepatic damage is unlikely to be idiosyncratic or due to hypersensitivity.
Outcome and Management
Niacin hepatotoxicity appears to be dose dependent and more common with the sustained release form of the drug. Hepatotoxicity is less common with regular, crystalline niacin or extended release niacin. Most cases are mild and resolve rapidly upon stopping the medication, although in some instances, the injury is acute and severe and progresses to liver failure that is fatal or requires emergency liver transplantation. Complete resolution of the clinical symptoms is expected within days of stopping niacin, whereas serum enzyme elevations may require several weeks or months to resolve. Rechallenge with the same form leads to rapid recurrence and should be avoided. If the injury occurred after switching to a SR formulation, the crystalline form of niacin may be restarted at a lower dose and with caution.
Drug Class: Antilipemic Agents; Vitamins
CASE REPORT
Case 1. Hepatocellular hepatitis with jaundice associated with high doses of niacin.(1)
A 41 year old man with a history of taking 4.5 grams of niacin daily for 6 months presented with nausea, anorexia, weakness and abdominal pain followed by jaundice. He was icteric but had no fever or rash. Blood tests showed marked elevations in serum aminotransferase levels and hyperbilirubinemia (Table). Tests for hepatitis B and for autoantibodies were negative. An initial prothrombin time was 28.5 seconds, but it corrected within 3 days of stopping niacin. A liver biopsy showed submassive lobular collapse, marked cholestasis, ballooned hepatocytes and mild fibrosis. Once niacin was stopped, he improved rapidly and serum enzymes fell to normal within a month. Review of this history revealed that he had developed jaundice while taking niacin in the past, had recovered on stopping, but nevertheless began taking it again.
Key Points
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 0 | Niacin started, gradually increasing to 4.5 g/day | ||||
| 20 weeks | Admission: niacin stopped | Nausea and jaundice | |||
| 20 weeks | 0 | 3300 | 379 | 7.2 | Protime 28.5 |
| 2 days | 2155 | 343 | 8.0 | ||
| 4 days | 1212 | 299 | 6.6 | Protime 12.5 | |
| 21 weeks | 9 days | 237 | 193 | 2.2 | Discharged |
| 6 months | 7 weeks | 35 | 78 | 0.7 | |
| 8 months | 12 weeks | 26 | 57 | 0.3 | |
| Normal Values | <41 | <115 | <1.2 | ||
Comment
This is a typical example of hepatotoxicity from high doses of niacin. The delayed latency was probably a result of the gradual increase in dose, the hepatic injury triggered by reaching critical levels. Serum aminotransferase levels were markedly elevated and there was a prompt improvement with stopping therapy. The prolongation of the prothrombin time indicated the severity and acuteness of the injury, and the biopsy confirmed this, showing submassive necrosis and collapse. Multiple such episodes with taking niacin can lead to posthepatitic cirrhosis. Note that if the patient had stopped niacin a week before being tested, serum aminotransferase elevations would be less impressive and the enzyme pattern would be considered mixed or cholestatic.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Niacin – Generic, Niacor®, Niaspan®
DRUG CLASS
Antilipemic Agents; Vitamins
Product labeling at DailyMed, National Library of Medicine, NIH
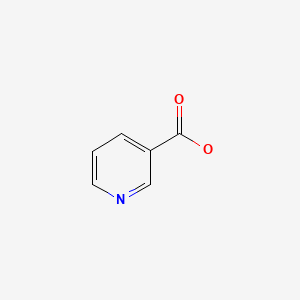
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Niacin | 59-67-6 | C6-H5-N-O2 |
|
CITED REFERENCE
- 1.
- Patterson DJ, Dew EW, Gyorkey F, Graham DY. Niacin hepatitis. South Med J. 1983;76:239–241. [PubMed: 6823602]
ANNOTATED BIBLIOGRAPHY
References updated: 09 September 2020
Abbreviations: ER, extended release; IR, immediate release; SR, sustained release.
- Zimmerman HJ. Drugs used in the treatment of hypercholesterolemia and hyperlipidemia. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 660-2.(Expert review of hepatotoxicity published in 1999; jaundice is reported in 3% of patients on nicotinic acid for a year or more; sustained release forms are more likely to cause injury; niacin may have direct, intrinsic hepatotoxicity).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic medications. Lipid lowering agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of lipid lowering agents; niacin is available over-the-counter and patients may take it in toxic amounts, >3 g daily of regular and lower amounts of sustained release niacin).
- Gurgle HE, Blumenthal DK. Drug therapy for dyslipidemias. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2013, pp. 605-18.(Textbook of pharmacology and therapeutics; niacin inhibits triglyceride synthesis which results in a decrease in hepatic LDL production and release).
- Rivin AU. Jaundice occurring during nicotinic acid therapy for hypercholesterolemia. J Am Med Assoc. 1959;170(17):2088–9. [PubMed: 13672808](23 year old man with familial hypercholesterolemia developed jaundice and pruritus after 18 months of niacin [3 g/day] therapy [bilirubin 7.4 mg/dL, AST 91 U/L, Alk P 3 times ULN], resolving within 20 days of stopping).
- Christensen NA, Achor RW, Berge KG, Mason HL. Nicotinic acid treatment of hypercholesteremia. Comparison of plain and sustained-action preparations and report of two cases of jaundice. JAMA. 1961;177:546–50. [PubMed: 13693364](Comparison of regular vs sustained release [SR] niacin found more adverse events and 2 cases of jaundice with SR form; 56 year old man tolerated regular niacin but developed abdominal pain and nausea after 4 days of the same dose of SR niacin [bilirubin 6.4 mg/dL, AST 20 times ULN, Alk P slightly elevated], resolving within 4 weeks of stopping; 51 year old man developed jaundice in first week of SR niacin therapy [bilirubin 3.5 mg/dL, AST 3 times and Alk P 3 times ULN], resolving in 7 weeks).
- Pardue WO. Severe liver dysfunction during nicotinic acid therapy. JAMA. 1961;175:137–8. [PubMed: 13732750](58 year old man developed edema 6 months after starting niacin [3 g daily] with bilirubin 1.3 mg/dL, AST 43 U/L, Alk P ~twice normal, albumin 2.0 g/dL, resolving in 3 months of stopping niacin).
- Parsons WB Jr. Studies of nicotinic acid use in hypercholesterolemia. Arch Intern Med. 1961;107:653–7. [PubMed: 13733026](Among 36 patients treated with niacin [3-6 g/day] for more than a year, 8 had increase in BSP retention and 5 had AST elevations which resolved on stopping; 3 case reports with cirrhosis in one and mild jaundice in two, resolving with stopping).
- Kohn RM, Montes M. Hepatic fibrosis following long acting nicotinic acid therapy: a case report. Am J Med Sci. 1969;258:94–9. [PubMed: 5805238](54 year old man found to have jaundice 4 years after starting niacin for hypercholesterolemia [bilirubin 11.8 mg/dL, ALT 59 U/L and Alk P 3 times ULN], resolving with stopping niacin; autopsy 2 years later showed posthepatitic cirrhosis).
- Winter SL, Boyer JL. Hepatic toxicity from large doses of Vitamin B (nicotinamide). N Engl J Med. 1973;289:1180–2. [PubMed: 4271091](35 year old man developed nausea and vomiting 18 months after starting nicotinamide for schizophrenia [bilirubin 5.6 mg/dL], resolving with stopping and recurring within 10 days of restarting excessively high doses [9 g/day] [bilirubin 4.9, ALT 2640 U/L]).
- Sugerman AA, Clark CG. Jaundice following the administration of niacin. JAMA. 1974;228:202. [PubMed: 4406053](69 year old man developed confusion and jaundice 18 months after starting niacin [750 mg/day] [bilirubin 8.3 rising to 19.6 mg/dL, AST 55 U/L, Alk P 205 U/L], worsening for 2 weeks and then resolving slowly over 6 months).
- Einstein N, Baker A, Galper J, Wolfe H. Jaundice due to nicotinic acid therapy. Am J Dig Dis. 1975;20:282–6. [PubMed: 1124751](23 year old woman developed jaundice 2.5 years after starting niacin [3 g/day] [bilirubin 30.4 mg/dL, ALT 136 U/L, Alk P 3 times ULN], resolving within 1 month of stopping).
- Patterson DJ, Dew EW, Gyorkey F, Graham DY. Niacin hepatitis. South Med J. 1983;76:239–41. [PubMed: 6823602](41 year old man developed recurrent jaundice on high doses of niacin [bilirubin 7.2 mg/dL, ALT 3300 U/L, Alk P 542 UL, prothrombin time 28.5 sec], resolving within 2 months of stopping: Case 1).
- Knopp RH, Ginsberg J, Albers JJ, Hoff C, Ogilvie JT, Warnick GR, Burrows E, Retzlaff B, Poole M. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: clues to mechanism of action of niacin. Metabolism. 1985;34(7):642–50. [PubMed: 3925290](Comparison of safety and efficacy of regular vs slow release [SR] niacin in 68 patients; flushing was less with SR form, but other side effects including AST and Alk P elevations were more common).
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality on coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–55. [PubMed: 3782631](Controlled trial of niacin vs placebo in 8341 men with coronary artery disease showed evidence of benefit in survival during long term follow up).
- Clementz GL, Holmes AW. Nicotinic acid-induced fulminant hepatic failure. J Clin Gastroenterol. 1987;9:582–4. [PubMed: 3680913](46 year old man developed nausea 1 month after starting niacin [3 g/day] [bilirubin 1.1 mg/dL, ALT 413 U/L], resolving with stopping, but recurrence with jaundice 10 weeks after restarting niacin [bilirubin 6.6 mg/dL, ALT 6220 U/L, Alk P 239 U/L, protime 90 sec], progressing over 3 days to stage 3 coma, but then rapid improvement, and all tests were normal 4 months later).
- Ferenchick G, Rovner D. Hepatitis and hematemesis complicating nicotinic acid use. Am J Med Sci. 1989;298:191–3. [PubMed: 2801756](20 year old man developed nausea and vomiting 7 days after starting high doses of niacin [6 g daily] [bilirubin 2.4 mg/dL, ALT 42→733 U/L, and protime of 17.6 sec], with rapid recovery on stopping).
- Mullin GE, Greenson JK, Mitchell MC. Fulminant hepatic failure after ingestion of sustained release nicotinic acid. Ann Intern Med. 1989;111:253–5. [PubMed: 2665592](44 year old man developed nausea, abdominal pain and fever 3 days after switching from regular niacin [6 g/day] to the same dose in a sustained release form [bilirubin 9.8 mg/dL, ALT 5186 U/L, Alk P 372 U/L, protime 64 sec], developing coma and undergoing liver transplant with uneventful recovery).
- Henkin Y, Johnson KC, Segrest JP. Rechallenge with crystalline niacin after drug-induced hepatitis from sustained-release niacin. JAMA. 1990;264:241–3. [PubMed: 2355446](Two men and one woman, ages 52 to 62 years, developed acute liver injury [bilirubin 0.8, 7.1 and 0.5 mg/dL, ALT 700, 870 and 55 U/L, Alk P 56, 2325 and 85 U/L] after 1-24 weeks of sustained release niacin, all three of whom then tolerated same dose [2-4 g/day] of regular, crystalline niacin for prolonged period).
- Hodis HN. Acute hepatic failure associated with the use of low-dose sustained release niacin. JAMA. 1990;264:181. [PubMed: 2355439](32 year old man developed nausea 2 months after starting SR niacin [500 mg daily] [bilirubin 11.9 mg/dL, ALT >9000 U/L, LDH 12,700 U/L and Alk P 209 U/L, prothrombin index <5%], progressing to hepatic coma, but full spontaneous recovery and normal liver tests 8 weeks later).
- Henkin Y, Oberman A. Hurst Dc, Segrest JP. Niacin revisited: clinical observations on an important but underutilized drug. Am J Med. 1991;91:239–46. [PubMed: 1892143](Retrospective analysis of 82 patients treated with niacin for dyslipidemia from 1987-90; none of 65 on regular niacin but 8 of 15 on sustained release niacin [4 different brands] developed liver injury, even in low doses; all but one with hepatocellular pattern of enzymes [ALT 810-970 U/L, Alk P 56-2325 U/L], one jaundiced, all recovered).
- Etchason JA, Miller TD, Squires RW, Allison TG, Gau GT, Martilla JK, Kottke BA. Niacin-induced hepatitis: a potential side effect with low-dose time-release niacin. Mayo Clin Proc. 1991;66:23–8. [PubMed: 1988755](Description of 5 cases of liver injury during sustained release [SR] niacin therapy [1.5 to 3 g/day] arising a few days to months after switching to SR or raising dose, all symptomatic but none jaundiced, all resolved promptly with stopping).
- Schwab RA, Bachhuber BH. Delirium and lactic acidosis caused by ethanol and niacin coingestion. Am J Emerg Med. 1991;9:363–5. [PubMed: 2054009](44 year old man with “toxic delirium” attributed to alcohol and niacin [3 g/day] [bilirubin 0.8 mg/dL, ALT 230 U/L, Alk P 96 U/L, lactic acid 9.5 mmol/L], with resolution of delirium despite continuing niacin and resolution of ALT elevations on decreasing niacin dose).
- Fischer DJ, Knight LL, Vestal RE. Fulminant hepatic failure following low-dose sustained release niacin therapy in hospital. West J Med. 1991;155:410–2. [PMC free article: PMC1003029] [PubMed: 1771885](56 year old man developed jaundice 6 days after hospitalization for bronchitis and administration of SR niacin [2 g/day] [bilirubin 6.0 mg/dL, ALT 834 U/L, Alk P 270 U/L], progressing to hepatic failure and death 4 days later).
- Rader JI, Calvert RJ, Hathcock JN. Hepatic toxicity of unmodified and time-release preparations of niacin. Am J Med. 1992;92:77–81. [PubMed: 1731514](Review from FDA of literature on hepatotoxicity from unmodified [mostly taking >3 g/day] and sustained release [SR] form of niacin [0.5-3 g/day]; in 10 cases, liver disease developed shortly after switching to SR form).
- Dalton TA, Berry RS. Hepatotoxicity associated with sustained-release niacin. Am J Med. 1992;93:102–4. [PubMed: 1626557](67 year old woman developed fatigue followed by agitation and stupor, starting 2 days after switching from regular to SR niacin [bilirubin 0.5 mg/dL, ALT 105 U/L, Alk P 446 U/L, protime 15.8 sec], resolving within 1 month of stopping).
- Dearing BD, Lavie CJ, Lohmann TP, Genton E. Niacin-induced clotting factor synthesis deficiency with coagulopathy. Arch Intern Med. 1992;152:861–3. [PubMed: 1558449](3 men, ages 44 to 57 years, on SR niacin for 1-3 months, developed coagulopathy with minimal liver test abnormalities [bilirubin 0.7-1.0 mg/dL, ALT 56-86 U/L, protime 17.2-19.9], with low triglyceride levels and recovery within 1-2 weeks upon stopping and recurrence on restarting SR niacin [1 patient], but not regular niacin [1 patient]).
- Lawrence SP. Transient focal hepatic defects related to sustained-release niacin. J Clin Gastroenterol. 1993;16:234–6. [PubMed: 8505497](37 year old woman developed fatigue and nausea 1 month after reaching SR niacin dose of 4 g/day [ALT 58 U/L, Alk P 503 U/L, bilirubin 0.8 mg/dL], but niacin continued and 3 months later CT showed 2 hypo-dense central defects in liver [interpreted as focal fat]; both enzyme elevations and imaging abnormality resolving with stopping niacin).
- Jacobson TA, Amorosa LF. Combination therapy with fluvastatin and niacin in hypercholesterolemia: a preliminary report on safety. Am J Cardiol. 1994;73:25D–29D. [PubMed: 8198020](Controlled trial of fluvastatin vs placebo followed by addition of niacin [up to 3 g/day] in 74 patients; ALT elevations occurred in 5.3% on fluvastatin and niacin compared to 6.5% of niacin alone; no case of clinically apparent liver injury and no elevation >3 times ULN).
- McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271:672–7. [PubMed: 8309029](Controlled trial of immediate- [IR] vs sustained release [SR] niacin for 6 weeks in 46 patients with hypercholesterolemia; SR form was less likely to cause flushing, but ALT elevations >3 times ULN occurred in 12 of 23 patients on 2-3 g/day of SR [5 symptomatic], but none of 23 on IR niacin).
- Coppola A, Brady PG, Nord HJ. Niacin-induced hepatotoxicity: Unusual presentations. South Med J. 1994;87:30–2. [PubMed: 8284714](Four cases of symptomatic hepatotoxicity during therapy with SR niacin for 3 or more months, with bilirubin 0.6-1.7 mg/dL, ALT 34-144 U/L, and Alk P 70 to 136 U/L, two had focal fat on CT scan, all resolved within 1-2 months).
- Patel SD, Taylor HC. Intrahepatic cholestasis during nicotinic acid therapy. Cleve Clin J Med. 1994;61:70–5. [PubMed: 8124850](61 year old man developed jaundice a year after starting regular niacin [3 g/day] [bilirubin 8.4 mg/dL, Alk P 1260, ALT 360 U/L], resolving within 6 weeks of stopping).
- Schwenk TL, Fisher M. Hepatitis caused by low-dose sustained-release niacin. J Am Board Fam Pract. 1994;7:242–4. [PubMed: 8059629](36 year old man developed severe fatigue 2 years after starting niacin and 2 months after switching to an SR form [500 mg/day] [bilirubin not given, ALT 556 U/L, Alk P 156 U/L], resolving within 5 weeks of stopping SR niacin).
- Reimund E, Ramos A. Niacin-induced hepatitis and thrombocytopenia after 10 years of niacin use. J Clin Gastroenterol. 1994;18:270–1. [PubMed: 8034946](32 year old man developed fatigue after taking niacin [3 g/day] for 3 years [bilirubin 1.2 mg/dL, ALT 139 U/L, Alk P 208 U/L], values returning to almost normal within 3 weeks of stopping).
- Brown WV. Niacin for lipid disorders: Indications, effectiveness, and safety. Postgrad Med. 1995;98:185–9, 192-3. [PubMed: 7630846](Review of efficacy and safety of niacin therapy of dyslipidemia; doses of niacin above 3 g per day and use of SR formulations are associated with higher rates of hepatotoxicity).
- Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA, Brody J. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82:74U–81U. [PubMed: 9915666](Open label study of ER niacin [1-3 g/day] in 517 patients for up to two years; flushing episodes in 75%, ALT elevations >twice ULN occurred in <1%, and none had levels >3 times ULN).
- Tatò F, Vega GL, Grundy SM. Effects of crystalline nicotinic acid-induced hepatic dysfunction on serum low-density lipoprotein cholesterol and lecithin cholesteryl acyl transferase. Am J Cardiol. 1998;81:805–7. [PubMed: 9527102](Three men, ages 46-66 years, developed mild nonspecific symptoms and hepatic dysfunction after 4 months of taking regular niacin with decrease in serum proteins, rise in prothrombin time and marked decline in cholesterol and triglycerides, but minimal increase in routine liver tests [bilirubin 0.6-1.2 mg/dL, ALT 16-73 U/L, Alk P 75-225 U/L], resolving within 4 weeks of stopping).
- Rourk RM, Rehman NU. Niacin. Help for your cholesterol--harm for your liver. N C Med J. 1998;59:87–8. [PubMed: 9558894](46 year old woman developed fatigue and jaundice a few weeks after reaching a dose of 10 g/day of SR niacin [bilirubin 6.2 mg/dL, ALT 2081 U/L, Alk P 295 U/], resolving within 3 weeks of stopping).
- Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82(12A):29U–34U. [PubMed: 9915660](Controlled trial of two doses of extended release niacin [ER: Niaspan] vs placebo in 96 patients; 83-88% had flushing episodes and 1 had ALT elevations >3 times ULN, but it resolved despite remaining on niacin).
- Kristensen T, Olcott EW. Effects of niacin therapy that simulate neoplasia: hepatic steatosis with concurrent hepatic dysfunction. J Comput Assist Tomogr. 1999;23:314–7. [PubMed: 10096345](52 year old man developed nausea 5 months after starting niacin [2 g/day] [bilirubin 1.6 mg/dL, ALT 110 U/L, Alk P 304 U/L] and hyperechoic lesions in liver by CT [interpreted as focal fat], resolving with stopping niacin).
- Scheer MS, Perlmutter S, Ross W, Katz DS. Ultrasonographic findings in niacin-induced hepatitis. J Ultrasound Med. 1999;18:321–3. [PubMed: 10206223](50 year old man developed abdominal pain 8 weeks after starting SR niacin [total daily dose 2 g/day] [bilirubin 0.9 mg/dL, AST 168 U/L, Alk P 97 U/L] and ultrasound showing hypoechoic lesions and “starry sky liver”).
- Kashyap ML, McGovern ME, Berra K, Guyton JR, Kwiterovich PO, Harper WL, Toth PD, et al. Long-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemia. Am J Cardiol. 2002;89:672–8. [PubMed: 11897208](Open label trial of the fixed combination of lovastatin [10 mg] and extended release [ER] niacin [500 mg] [Advicor] escalating to 4 daily in 814 patients; 4 [0.5%] had ALT elevations >3 times ULN and 3 were withdrawn for abnormal liver tests, but none were clinically apparent).
- Gavilán JC, Bermúdez FJ, Belmonte A, González-Santos P. Med Clin (Barc). 2002;118:558. [Niacin-induced hepatitis] [PubMed: 11988158](50 year old man developed jaundice, pruritus and fatigue during the 6th year of taking niacin in doses of 2.5-3.5 g/day [bilirubin 5.3 mg/dL, ALT 119 U/L, Alk P 781 U/L, prothrombin index 36%], resolving within 8 weeks of stopping).
- Bays HE, Dujovne CA, McGovern ME, White TE, Kashyap ML, Hutcheson AG, Crouse JR., Advicor Versus Other Cholesterol-Modulating Agents Trial Evaluation. Comparison of once-daily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin (the Advicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]). Am J Cardiol. 2003;91:667–72. [PubMed: 12633795](Controlled trial comparing lovastatin combined with niacin to atorvastatin or simvastatin alone in 315 patients for 16 weeks; no patient had confirmed ALT elevation >3 times ULN).
- Pieper JA. Overview of niacin formulations: differences in pharmacokinetics, efficacy, and safety. Am J Health Syst Pharm. 2003;60:S9–14. [PubMed: 12901025](Review of pharmacology, efficacy and safety of niacin as a lipid lowering agent; the extended release [ER] form has intermediate rates of dissolution and absorption between regular and sustained release [SR] niacin and appears to have lower rates of flushing compared to regular and less hepatotoxicity compared to SR forms).
- Zhao XQ, Morse JS, Dowdy AA, Heise N, DeAngelis D, Frohlich J, Chait A, et al. Safety and tolerability of simvastatin plus niacin in patients with coronary artery disease and low high-density lipoprotein cholesterol (The HDL Atherosclerosis Treatment Study). Am J Cardiol. 2004;93:307–12. [PubMed: 14759379](Controlled trial of simvastatin and niacin vs placebo in 160 patients for up to 3 years; side effects were similar in the two groups with AST elevations >3 times ULN in 2 patients on simvastatin/niacin and one on placebo).
- Alsheikh-Ali AA, Karas RH. Safety of lovastatin/extended release niacin compared with lovastatin alone, atorvastatin alone, pravastatin alone, and simvastatin alone(from the United States Food and Drug Administration adverse event reporting system). Am J Cardiol. 2007;99:379–81. [PubMed: 17261402](Analysis of MedWatch reports of adverse events found no excess in liver related adverse event reports per million prescriptions of lovastatin alone [2.3] vs niacin alone [2.5] vs the combination [3.2], but slightly higher rates with atorvastatin [4.5], simvastatin [5.7] and pravastatin [4.9], but data are based upon spontaneous reporting).
- Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–613. [PMC free article: PMC2048990] [PubMed: 17723922](Review of hepatotoxicity of lipid lowering agents; injury from niacin is most common with sustained release forms; onset of injury ranges from 1 week to 2 years after starting drug, usually subsiding rapidly with stopping; acute liver failure occurs, but is rare).
- Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007;99:22C–31C. [PubMed: 17368274](Expert review of safety of niacin; in contrast to the over-the-counter sustained release forms, the prescription requiring extended release form of niacin has not been associated with a higher rate of hepatotoxicity).
- Birjmohun RS, Kastelein JJ, Poldermans D, Stroes ES, Hostelek U, Assman G. Safety and tolerability of prolonged-release nicotinic acid in statin-treated patients. Curr Med Res Opin. 2007;23:1707–13. [PubMed: 17588301](Open label study of combination of extended release niacin [Niaspan] added to preexisting statin therapy for 6 months in 1053 patients; flushing in 40%, no significant change in AST or ALT and no clinically apparent hepatotoxicity).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, only one case was attributed to niacin: 53 year old man developed nausea and fatigue 2 months after starting SR niacin [bilirubin 2.3 mg/dL, ALT 595 U/L, Alk P 132 U/L], resolving within 2 weeks of stopping).
- Bays H. Safety of niacin and simvastatin combination therapy. Am J Cardiol. 2008;101(8A):3B–8B. [PubMed: 18375239](Review of safety of the combination of niacin and simvastatin; ALT elevations associated mostly with slow release [SR] rather than the extended [ER] or intermediate [IR] release formulations of niacin).
- Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51:1564–72. [PubMed: 18420099](Controlled trial comparing niacin alone vs simvastatin/ezetimibe vs the triple combination for 24 weeks in 1220 patients with hypercholesterolemia; confirmed ALT elevations >3 times ULN occurred in 0.4% on niacin, 0.4% on simvastatin/ezetimibe and 0.5% on all three).
- Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–26B. [PubMed: 18375237](Niacin inhibits hepatocyte diacylglycerol acyltransferase-2, an important enzyme in triglyceride synthesis, making triglycerides less available for uptake into lipoprotein particles and resulting in accelerated degradation of apo B and decreased secretion of VLDL and LDL particles by the liver).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to niacin or nicotinamide).
- McKenney J, Bays H, Koren M, Ballantyne CM, Paolini JF, Mitchel Y, Betteridge A, et al. Safety of extended-release niacin/laropiprant in patients with dyslipidemia. J Clin Lipidol. 2010;4:105–112.e1. [PubMed: 21122637](Analysis of results from 4747 patients enrolled in 6 large studies of ER niacin with or without laropiprant [which decreases niacin related flushing] compared to simvastatin or placebo; ALT or AST elevations >3 times ULN occurred in 0.5%-1% of niacin recipients compared to 0.9% on simvastatin, and no patient developed "treatment related hepatitis").
- Byrd C, Mowrey KA. Lipid and transaminase concentrations after formulary conversion of Niaspan to Slo-Niacin. Am J Health Syst Pharm. 2010;67:2038–42. [PubMed: 21098376](Retrospective analysis of effects of converting 142 patients from a prescription ER niacin [Niaspan] to an over-the-counter SR niacin [Slo-Niacin]; ALT levels increased in 31% of patients, but none rose to above 3 times ULN).
- Lavie CJ, Milani RV. Efficacy and safety of sustained-release niacins. Am J Health Syst Pharm. 2011;68(14):1294–1297. [PubMed: 21719588](Editorial regarding Byrd [2010] stressing the lack of safety of some formulations of SR-niacin).
- AIM-HIGH Investigators. Boden WE, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. [PubMed: 22085343](3414 patients with cardiovascular disease were treated with statins and ezetimibe and either ER niacin or placebo and followed for cardiovascular outcomes; niacin use was associated with a rise in HDL cholesterol and fall in triglycerides levels, but clinical outcomes were not affected; adverse events were rare with liver test abnormalities arose in 0.5% of placebo and 0.8% of niacin recipients).
- Aramwit P, Srisawadwong R, Supasyndh O. Effectiveness and safety of extended-release nicotinic acid for reducing serum phosphorus in hemodialysis patients. J Nephrol. 2012;25:354–62. [PubMed: 21748722](Among 28 hemodialysis patients with hyperphosphatemia randomized to receive ER niacin [0.5-1 g/day] or placebo for 12 weeks, no patient developed significant serum enzyme elevations).
- Dunatchik AP, Ito MK, Dujovne CA. A systematic review on evidence of the effectiveness and safety of a wax-matrix niacin formulation. J Clin Lipidol. 2012;6:121–31. [PubMed: 22385545](Analysis of 4 published studies of SR niacin [Endur-acin] in 635 hyperlipidemic patients treated for 8-38 weeks, showed mild, overall changes in Alk P and AST levels, but none rose above 1.6 times baseline values; one patient developed anicteric but symptomatic hepatitis that resolved upon stopping niacin).
- Ali EH, McJunkin B, Jubelirer S, Hood W. Niacin induced coagulopathy as a manifestation of occult liver injury. W V Med J. 2013;109:12–4. [PubMed: 23413541](61 year old man was found to have abnormal coagulation studies with minimal ALT elevations, having been on ER niacin for 4 years [INR 1.5, platelets 117,000/μL, bilirubin 1.4 mg/dL, ALT 44 U/L], resolving within 4 weeks of stopping).
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91. [PMC free article: PMC3640201] [PubMed: 23444397](Among 25,673 subjects stabilized on statin therapy and then randomized to receive niacin with laropiprant versus placebo, serum ALT elevations above 3 times ULN occurred in 0.30% of patients on niacin vs 0.14% on placebo per year and occurrence of ALT elevations with jaundice was rare [<0.01%]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to niacin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attribute to niacin).
- HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. [PubMed: 25014686](Among 25,673 adults with vascular disease treated with statins with or without niacin combined with laropiprant [to decrease flushing reactions] for a median of 3.9 years, LDL cholesterol levels were lower with niacin [by 10 mg/dL] and HDL levels were higher [by 6 mg/dL] but rates of vascular events were similar [13.2% vs 13.7%], while side effects were more common particularly diabetes and myalgias [but not hepatic events]).
- Anderson TJ, Boden WE, Desvigne-Nickens P, Fleg JL, Kashyap ML, McBride R, Probstfield JL. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288–90. [PMC free article: PMC4156937] [PubMed: 25014706](Editorial in response to HPS2-THRIVE study [2014] mentions that side effects of niacin including myopathy, bleeding and glucose elevations were also seen in the AIM-HIGH trial [2011]).
- Lloyd-Jones DM. Niacin and HDL cholesterol--time to face facts. N Engl J Med. 2014;371:271–3. [PubMed: 25014692](Editorial in response to HPS2-THRIVE study [2014] concludes that niacin has “an unacceptable toxicity profile for the majority of patients, and it should not be used routinely”).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, United States Drug Induced Liver Injury Network. et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 363 [36%] were attributed to antibiotics, 5 [0.6%] of which were attributed to niacin).
- McKenney J, Bays H, Gleim G, Mitchel Y, Kuznetsova O, Sapre A, Sirah W, et al. Safety and tolerability of extended-release niacin-laropiprant: pooled analyses for 11,310 patients in 12 controlled clinical trials. J Clin Lipidol. 2015;9:313–25. [PubMed: 26073389](Analysis of 12 clinical studies with 12,310 patients treated for 12-52 weeks with niacin/laropiprant or niacin alone or a statin for rates of adverse events indicated that episodes of bleeding and myopathy were no more frequent with niacin/laropiprant than niacin alone and ALT elevations occurred at similar rates in all 3 groups: 1.4% vs 1.0% vs 1.5%).
- Schaffellner S, Stadlbauer V, Sereinigg M, Mìller H, Högenauer C, Fickert P, Krumnikl J, et al. Niacin-associated acute hepatotoxicity leading to emergency liver transplantation. Am J Gastroenterol. 2017;112:1345–6. [PubMed: 28766583](22 year old female athlete took a single dose of 20 gm of native niacin and developed acute liver failure within 3 days [bilirubin 3.6 mg/dL, ALT 12,594 U/L, Alk P 165 U/L, INR above 8.9], undergoing successful liver transplantation within 12 hours).
- Durham SH, Covington EW, Clemmons KJ. Hepatotoxicity upon using niacin to pass a drug test: A case report. Journal of the American Pharmacists Association. 2018;58(5):564–7. [PubMed: 29941333](25 year old man with HIV infection on long term antiretroviral therapy was found to have elevated serum aminotransferase levels without jaundice on a routine clinic visit [ALT 158 U/L, AST 982 U/L], which rapidly resolved at which point he admitted to taking large doses of niacin to mask a positive urine test for marijuana use done for a job interview).
- Lipid-lowering drugs. Med Lett Drugs Ther. 2019;61(1565):17–24. [PubMed: 30845106](Concise summary of drugs for treating hypercholesterolemia states that there is no evidence that niacin improves cardiovascular outcomes and it has troublesome adverse events including hepatotoxicity).
- Haynes R, Valdes-Marquez E, Hopewell JC, Chen F, Li J, Parish S, Landray MJ, HPS2-THRIVE Collaborative Group. HPS2-THRIVE Writing Committee members. HPS2-THRIVE Steering Committee members. et al. Serious adverse effects of extended-release niacin/Laropiprant: results from the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) Trial. Clin Ther. 2019;41:1767–77. [PubMed: 31447131](Reanalysis of the adverse events that arose in the HPS2-THRIVE study [2014] for risk factors for the excess in bleeding episodes, infection and diabetes found no specific risk factors; no analysis of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Targeting low HDL-cholesterol to decrease residual cardiovascular risk in the managed care setting.[J Manag Care Pharm. 2008]Review Targeting low HDL-cholesterol to decrease residual cardiovascular risk in the managed care setting.Cziraky MJ, Watson KE, Talbert RL. J Manag Care Pharm. 2008 Oct; 14(8 Suppl):S3-28; quiz S30-1.
- Review The effects of niacin on lipoprotein subclass distribution.[Prev Cardiol. 2004]Review The effects of niacin on lipoprotein subclass distribution.Morgan JM, Carey CM, Lincoff A, Capuzzi DM. Prev Cardiol. 2004 Fall; 7(4):182-7; quiz 188.
- Effect of drugs on high-density lipoprotein.[J Clin Lipidol. 2007]Effect of drugs on high-density lipoprotein.McKenney JM. J Clin Lipidol. 2007 Mar; 1(1):74-87. Epub 2007 Feb 15.
- Review The therapeutic role of niacin in dyslipidemia management.[J Cardiovasc Pharmacol Ther. 2...]Review The therapeutic role of niacin in dyslipidemia management.Boden WE, Sidhu MS, Toth PP. J Cardiovasc Pharmacol Ther. 2014 Mar; 19(2):141-58. Epub 2013 Dec 20.
- Review Niacin as a component of combination therapy for dyslipidemia.[Mayo Clin Proc. 2003]Review Niacin as a component of combination therapy for dyslipidemia.Miller M. Mayo Clin Proc. 2003 Jun; 78(6):735-42.
- Niacin - LiverToxNiacin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...